Method for preparing dimethyl ether, low carbon olefin hydrocarbon with combination of methanol dehydration catalytic pyrolysis
A catalytic cracking and methanol dehydration technology, which is applied in the production of hydrocarbons from oxygen-containing organic compounds, dehydration of hydroxyl-containing compounds to prepare ethers, ether preparations, etc., can solve the problems of low coke formation rate, reduction, and alkane content reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The purity of methanol in the methanol feedstock is 99.5% by weight, and the hydrocarbon feedstock is vacuum gas oil (VGO), the properties of which are shown in Table 1. The code name of the catalyst used in this example is MTD-1 (containing 20% by weight of ZSM-5 molecular sieve, 10% by weight of USY molecular sieve, and the balance as a carrier, all based on the total weight of the catalyst).
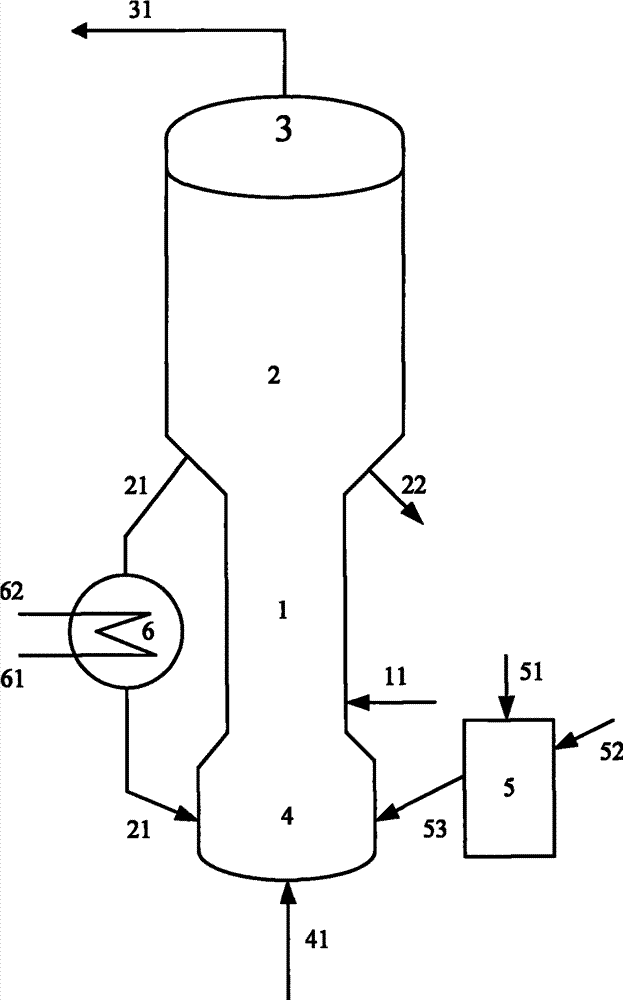
[0058] The gaseous methanol feedstock enters the fluidized bed reactor and contacts with the MTD-1 catalyst. It reacts at a temperature of 280°C, a pressure of 0.6MPa, a weight ratio of catalyst to methanol feedstock (agent to alcohol ratio) of 30, and a reaction time of 8.1 seconds. The stream is separated to obtain a product stream composed mainly of coke catalyst and dimethyl ether. The composition of the material is shown in Table 2. The material is directly sent to the stripper of the catalytic cracking unit without separation to react with the hot catalyst. The coke deposi...
Embodiment 2
[0062] The purity of methanol in the methanol feedstock is 95% by weight, and the hydrocarbon feedstock is vacuum gas oil (VGO), whose properties are shown in Table 1. The code name of the catalyst used in this example is MTD-2 (containing 15% by weight SAPO molecular sieve, 10% by weight ZSM-5 molecular sieve, 10% by weight USY molecular sieve, the balance is the carrier, all based on the total weight of the catalyst).
[0063] The gaseous methanol feedstock enters the fluidized bed reactor and contacts with the MTD-1 catalyst, and reacts under the conditions of a temperature of 320°C, a pressure of 0.3MPa, a weight ratio of catalyst to methanol feedstock (reagent ratio) of 2.5, and a reaction time of 3.2 seconds. The stream is separated to obtain a product stream composed mainly of coke catalyst and dimethyl ether. The composition of the material is shown in Table 2. The material is directly sent to the stripper of the catalytic cracking unit without separation to react with the...
Embodiment 3
[0067] The purity of methanol in the methanol feedstock is 99.5% by weight, and the hydrocarbon feedstock is vacuum gas oil (VGO), the properties of which are shown in Table 1. The code name of the catalyst used in this example is MTD-1 (containing 20% by weight of ZSM-5 molecular sieve, 10% by weight of USY molecular sieve, and the balance as a carrier, all based on the total weight of the catalyst).
[0068] The gaseous methanol feedstock enters the fluidized bed reactor and contacts with the MTD-1 catalyst. It reacts under the conditions of a temperature of 360°C, a pressure of 0.4MPa, a weight ratio of catalyst to methanol feedstock (agent to alcohol ratio) of 1, and a reaction time of 5.4 seconds. The stream is separated to obtain a product stream composed mainly of coke catalyst and dimethyl ether. The composition of the material is shown in Table 2. The material is directly sent to the bed reactor of the catalytic cracking unit without separation to react with the hot cat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com