Method for producing dimethyl ether by catalytic cracking coupling methanol dehydration
A catalytic cracking and methanol dehydration technology, which is applied to the preparation of ether by dehydration of hydroxyl-containing compounds, chemical instruments and methods, molecular sieve catalysts, etc., can solve the problems of insufficient heat balance and large heat of vaporization of methanol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
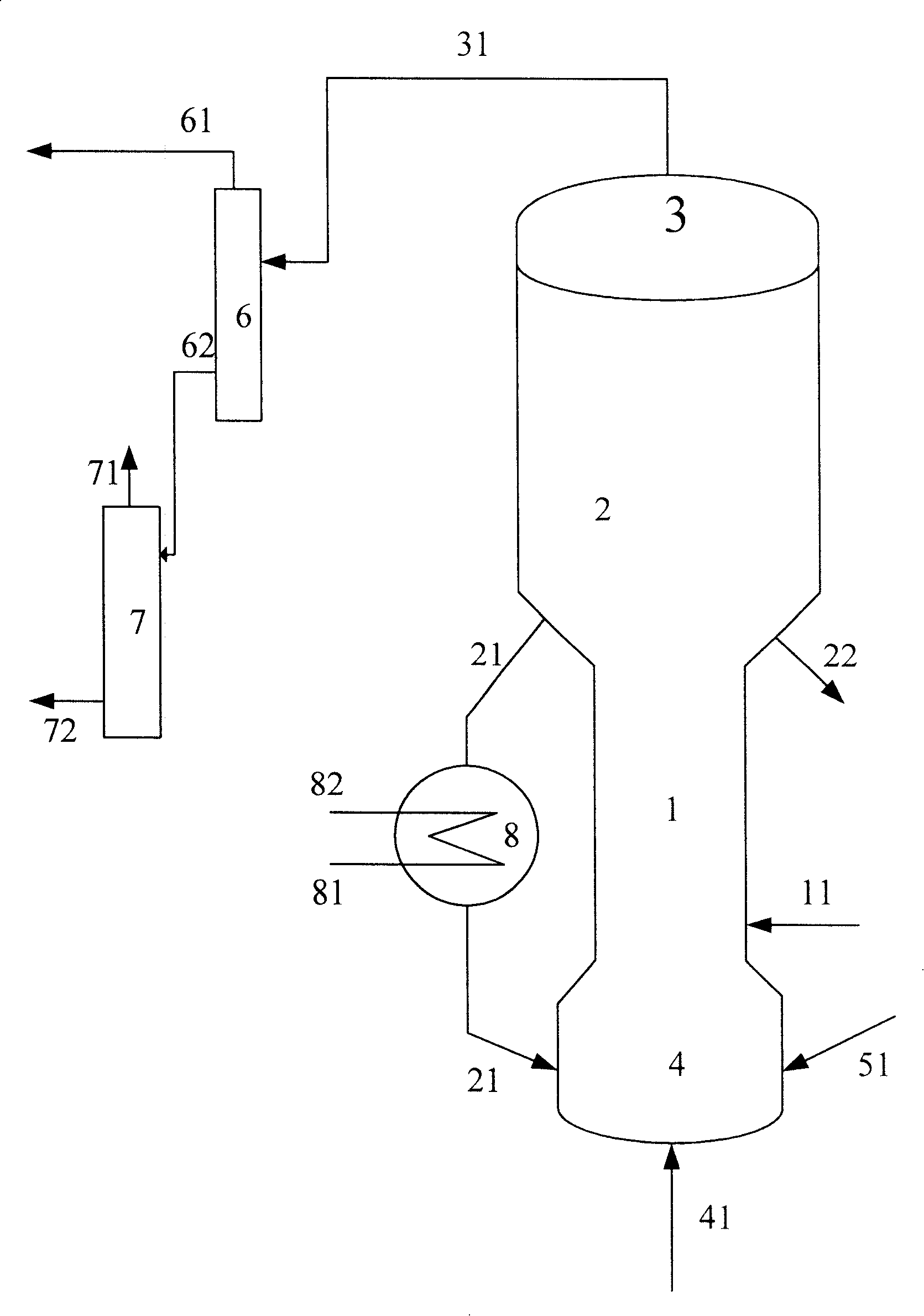
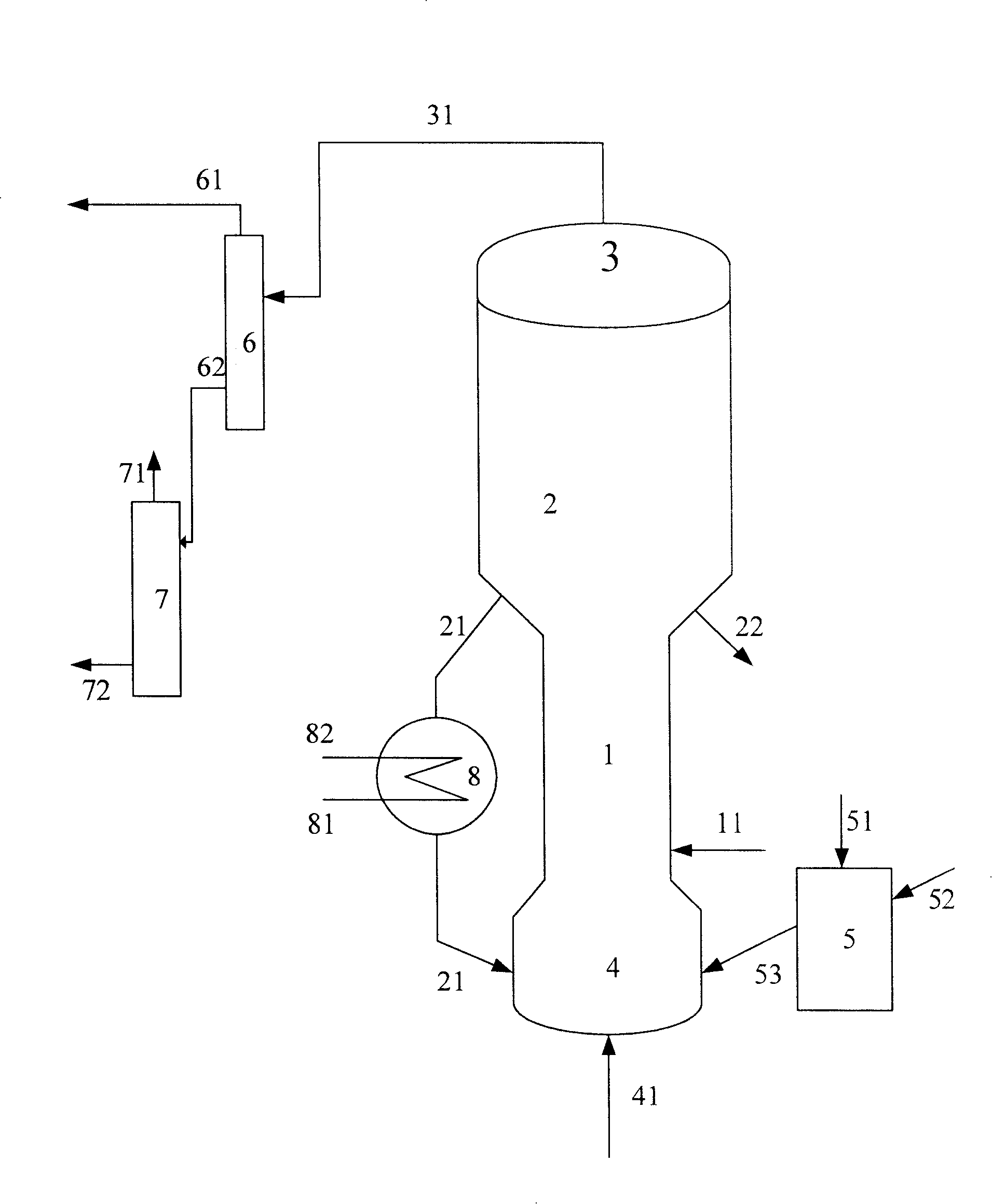
[0055] The purity of methanol in the methanol raw material is 99.5% by weight, and the hydrocarbon raw material is vacuum gas oil (VGO), and its properties are shown in Table 1. The code name of the catalyst used in this example is MTD-1 (contains 30% by weight of USY zeolite, 5% by weight of ZSM-5 zeolite, and the rest is carrier, all based on the total weight of the catalyst).
[0056] The gaseous methanol raw material enters the combined reactor of the short riser and the fluidized bed to contact with the fresh MTD-1 catalyst. At a temperature of 250° C. and a pressure (gauge pressure) of 0.1 MPa, the weight ratio (agent-alcohol ratio) of the catalyst to the methanol raw material is 2.5, weight hourly space velocity 3.0h -1 Reaction under the conditions, reactant flow is separated to obtain carbon deposition catalyst and product flow, and this product flow is further separated to obtain the target product dimethyl ether, product distribution is as shown in table 2, and exce...
Embodiment 2
[0061] The purity of methanol in the methanol raw material is 90.0% by weight, and the hydrocarbon raw material is VGO, and its properties are shown in Table 1. The code name of the catalyst used in this example is MTD-2 (containing 35% by weight of USY zeolite, and the balance is a carrier, all based on the total weight of the catalyst)
[0062] The gaseous methanol raw material enters the methanol reactor and contacts with the MTD-2 catalyst, at a temperature of 280°C, a pressure (gauge pressure) of 0.1MPa, a weight ratio of catalyst to methanol raw material (agent-alcohol ratio) of 0.5, and a weight hourly space velocity of 20h -1 Reaction under the conditions, reactant flow is separated to obtain carbon deposition catalyst and product flow, and this product flow is further separated to obtain the target product dimethyl ether, product distribution is as shown in table 2, and excess methanol is returned to methanol reactor; carbon deposition catalyst is divided into Two par...
Embodiment 3
[0067] The purity of methanol in the methanol raw material is 95.0% by weight, and the hydrocarbon raw material is VGO, and its properties are shown in Table 1. The code name of the catalyst used in this example is MTD-3 (containing 30% by weight of USY zeolite, 5% by weight of Beta zeolite, and the balance being carrier, all based on the total weight of the catalyst).
[0068] The gaseous methanol raw material enters the methanol reactor and contacts with the MTD-3 catalyst. At a temperature of 230°C and a pressure (gauge pressure) of 0.1MPa, the weight ratio of the catalyst to the methanol raw material (agent-alcohol ratio) is 6, and the weight hourly space velocity is 0.1h -1 Reaction under the conditions, reactant flow is separated to obtain carbon deposition catalyst and product flow, and this product flow is further separated to obtain the target product dimethyl ether, product distribution is as shown in table 2, and excess methanol is returned to methanol reactor; carbo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com