Novel taxane halogenation derivates with anti-tumor activity
An anti-tumor activity and taxane technology, applied in the field of biomedicine, can solve the problems of high systemic toxicity, multidrug resistance of tumor cells, narrow treatment threshold, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0093] Example 1
[0094] Preparation of compounds DCB6101, DCB6102, DCB6103, DCB6104
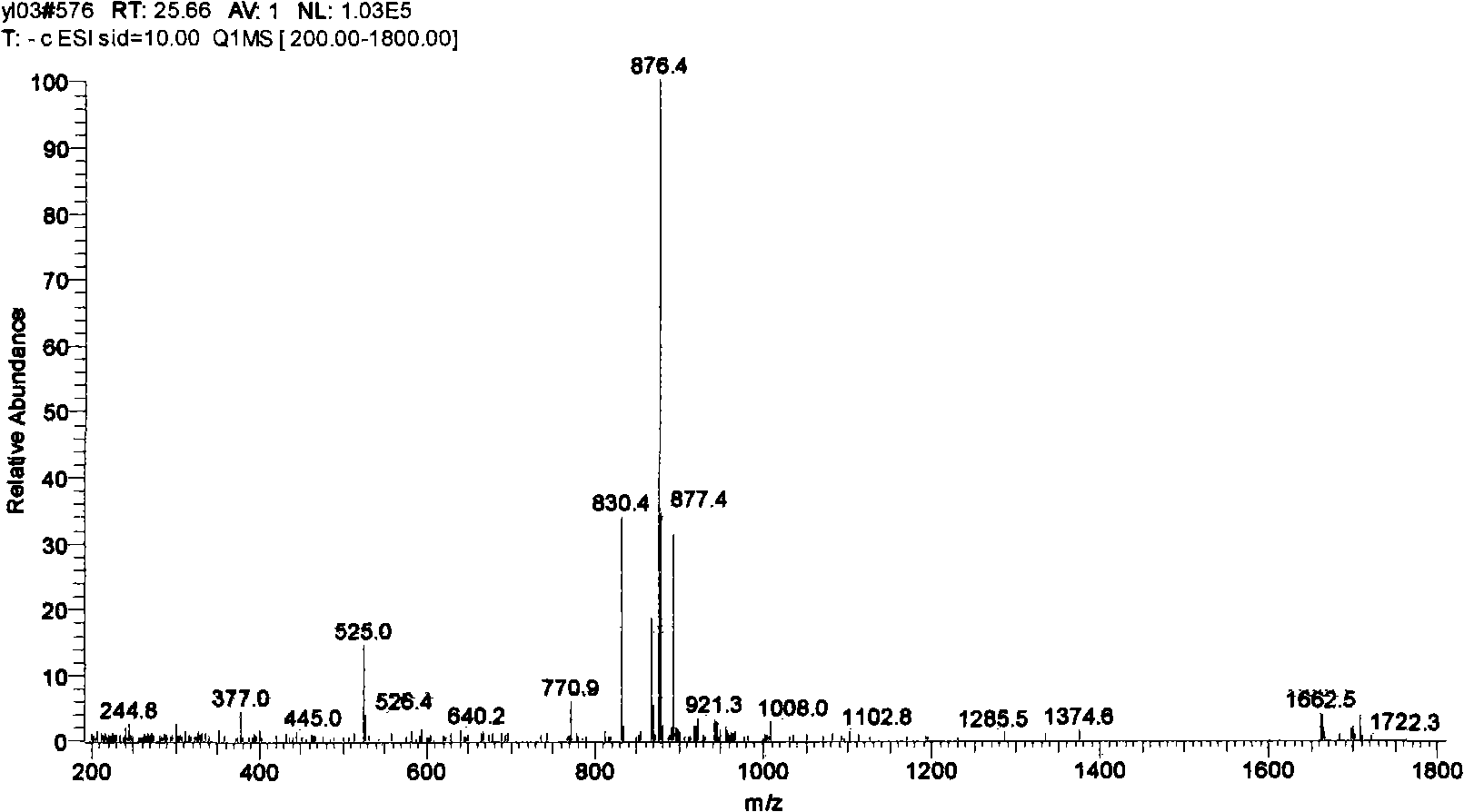
[0095] Weigh 1g taxane sample (determined by HPLC external standard method, containing 17% paclitaxel, 71% cephalomannine, see the LC-MS spectrum figure 1 ) Dissolve in 4ml acetonitrile system, add 1ml deionized water, 250mg NBS, and react at room temperature for 3 hours. HPLC detection shows that the conversion of cephalomannine is complete. The product was directly dried by rotary evaporation, and then subjected to silica gel column chromatography to obtain 760 mg of 2"-bromo-3"-hydroxy-cephalomannine, with a content of about 72%. After the partial products were separated by C18 reverse phase preparative chromatography, 530 mg of 2"-bromo-3"-hydroxy-cephalomannine diastereomer mixture was obtained, the purity was about 83%, and the total yield was 55.5%. It is configured into a 10mg / ml methanol solution and passed through a reversed-phase (YMC, C18 column) high performance liquid chromatogra...
Example Embodiment
[0097] Example 2
[0098] Preparation of compounds DCB6109, DCB6110, DCB6111, DCB6112
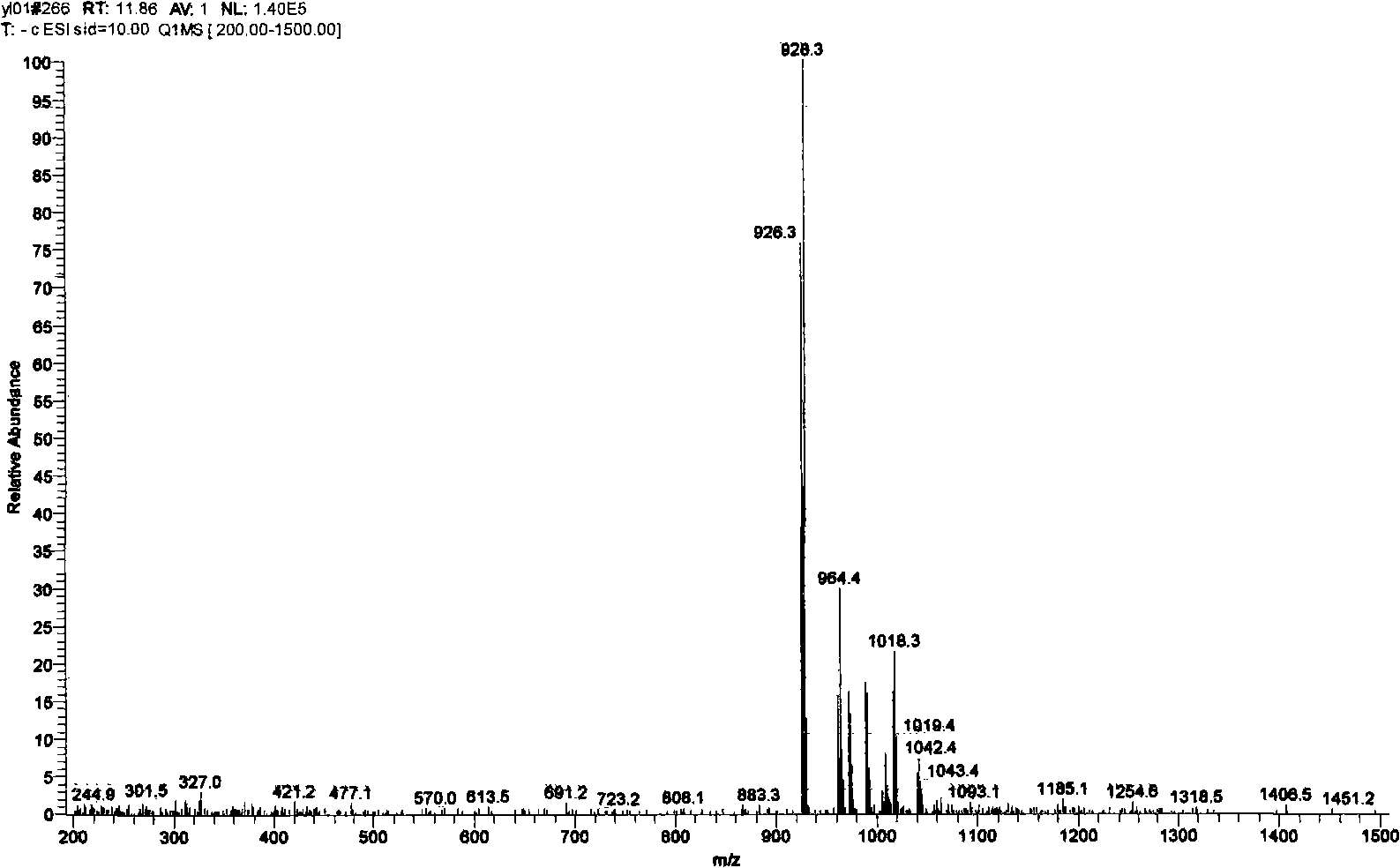
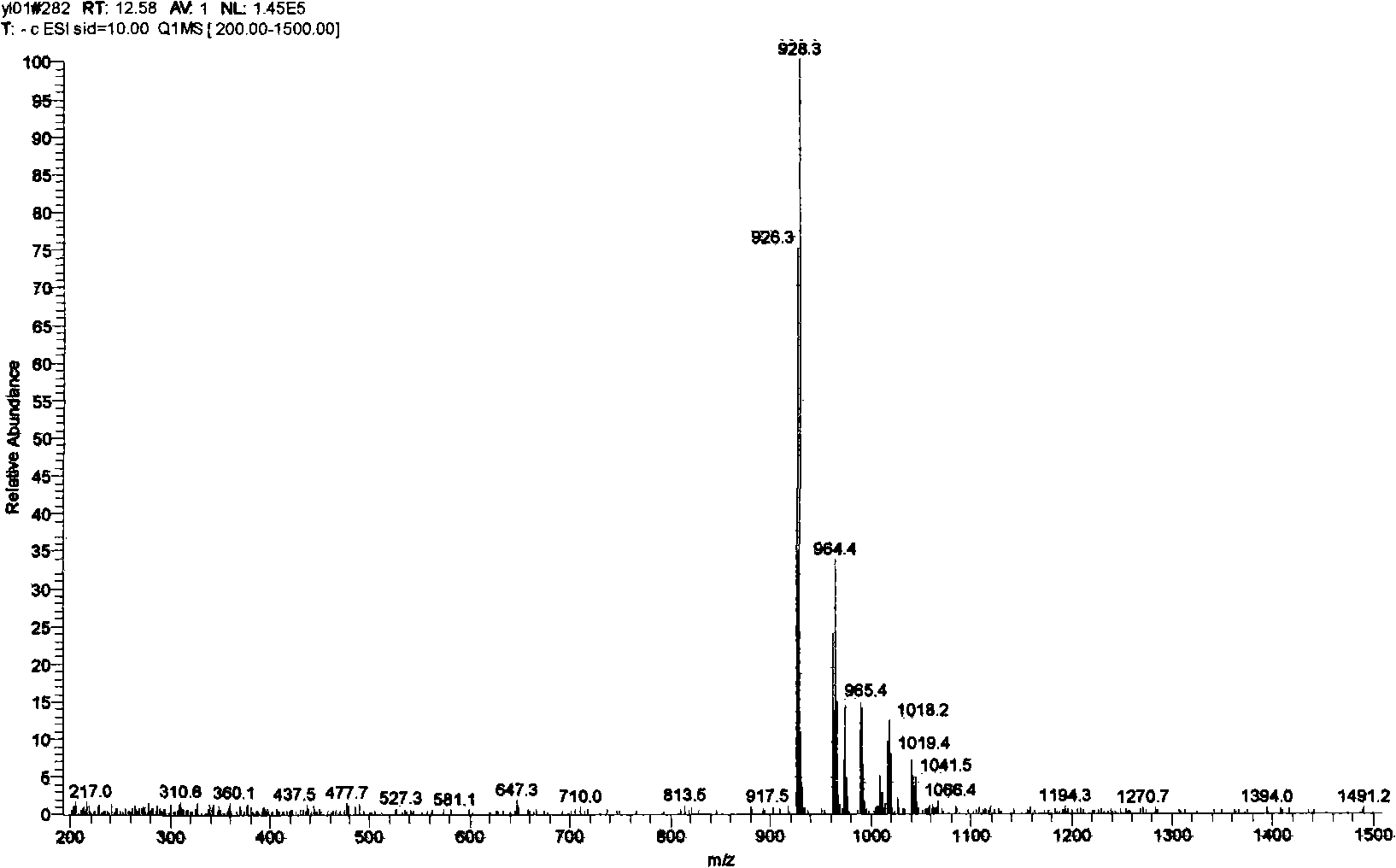
[0099] Take 10-desacetyl-cephalomannine (content 65%, LC-MS spectrum see Figure 4 ) Is the raw material for the reaction, and the synthesis method is similar to that in Example 1, and the separation and purification steps for the preparation of the diastereoisomer mixture will not be repeated. Reversed phase (YMC, C18 column) high performance liquid chromatography system was used to complete the resolution of diastereomers. The mixture of different diastereomers was prepared into a methanol solution of 10 mg / ml, and the mobile phase of water: acetonitrile (65:35) was used to rinse at a flow rate of 1 ml / min to obtain compound DCB6109 (content 93%). Compound DCB6110 (content 91%), compound DCB6111 (content 95%), compound DCB6112 (content 95%).
Example Embodiment
[0100] Example 3
[0101] Preparation of compounds DCB6105, DCB6106, DCB6107, DCB6108
[0102]Weigh 1g taxane sample (determined by HPLC external standard method, containing 17% paclitaxel, 71% cephalomannine) and dissolve it in 5ml methanol system, add 50mg ytterbium triflate, 250mg NBS, and react at room temperature for 1 Hours, HPLC detection showed that the conversion of cephalomannine was complete. The product was directly dried by rotary evaporation, and then subjected to silica gel column chromatography to obtain 680 mg of 2"-bromo-3"-methoxy-cephalomannine, with a content of about 77%. After the partial product was separated by C18 reversed-phase preparative chromatography, 510 mg of 2"-bromo-3"-methoxy-cephalomannine diastereomer mixture was obtained, the purity was higher than 90%, and the total yield was 56 %. It is configured into a 10mg / ml methanol solution and washed with a mobile phase of water: acetonitrile (45:55) at a flow rate of 1ml / min to obtain 33mg of compou...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap