Novel triptan formulations and methods for making them
A lubricant and disintegrant technology, applied in the field of orally disintegrating tablets, can solve problems such as dull gloss, unsuitable taste, and reduced actual value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
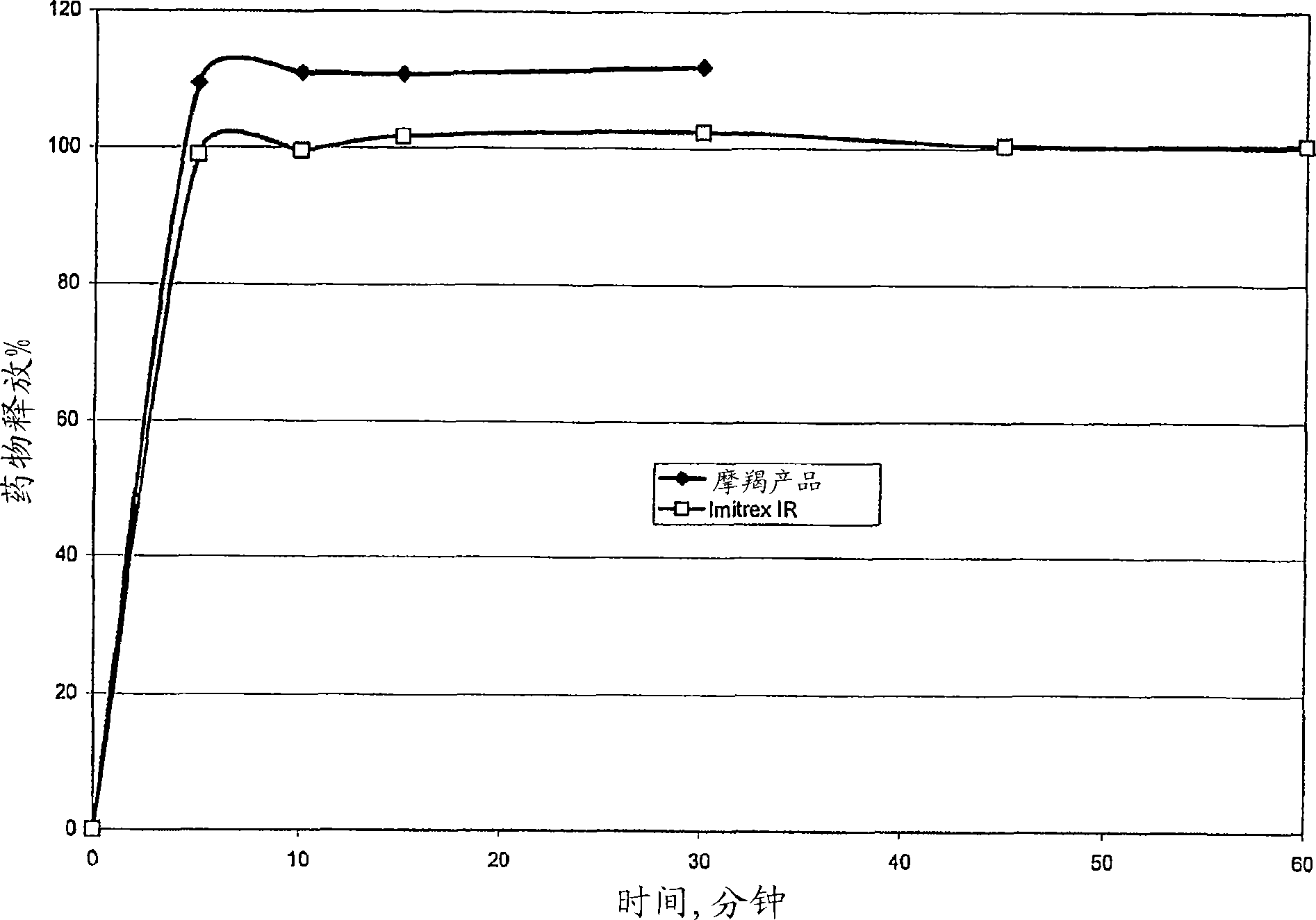
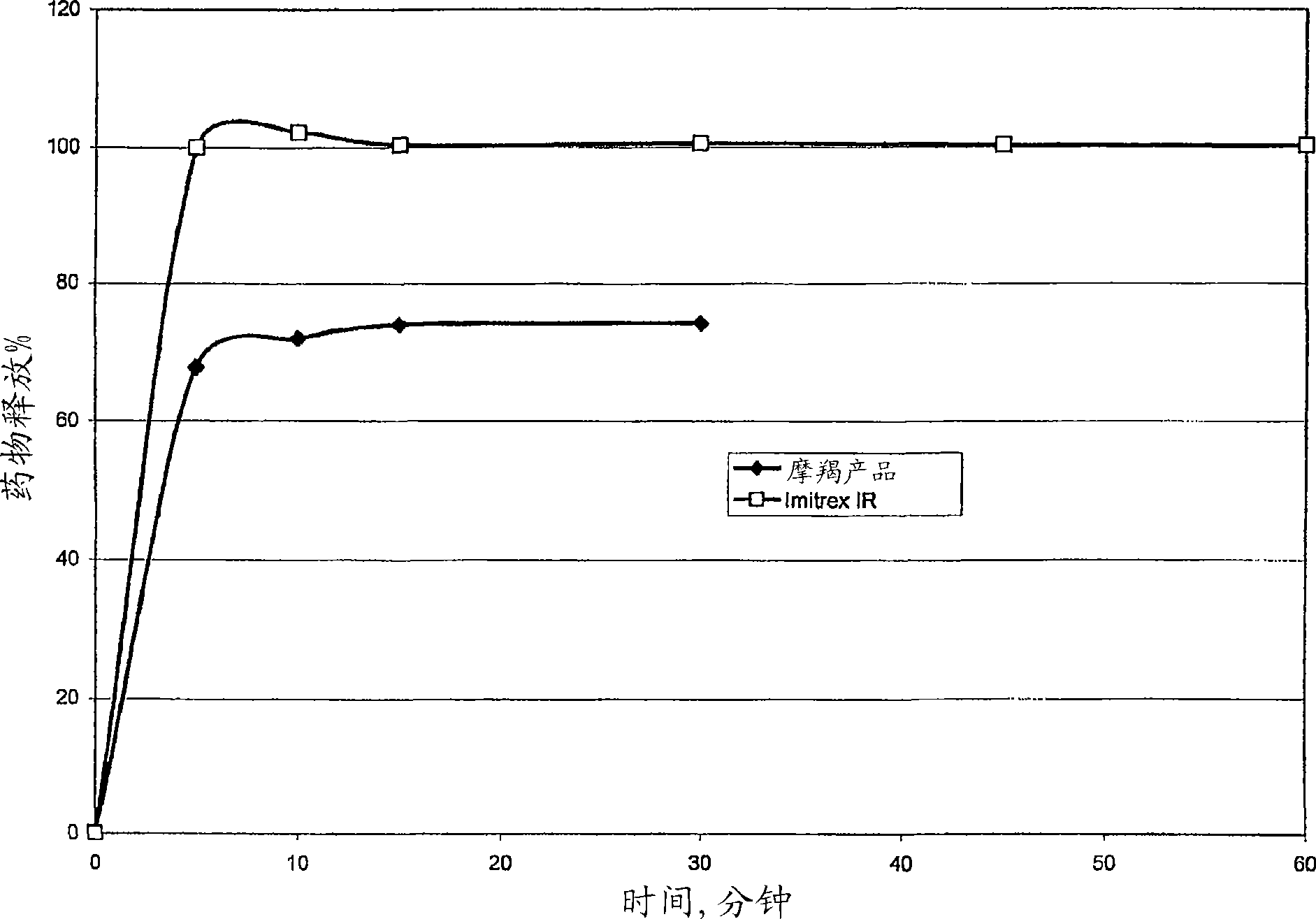
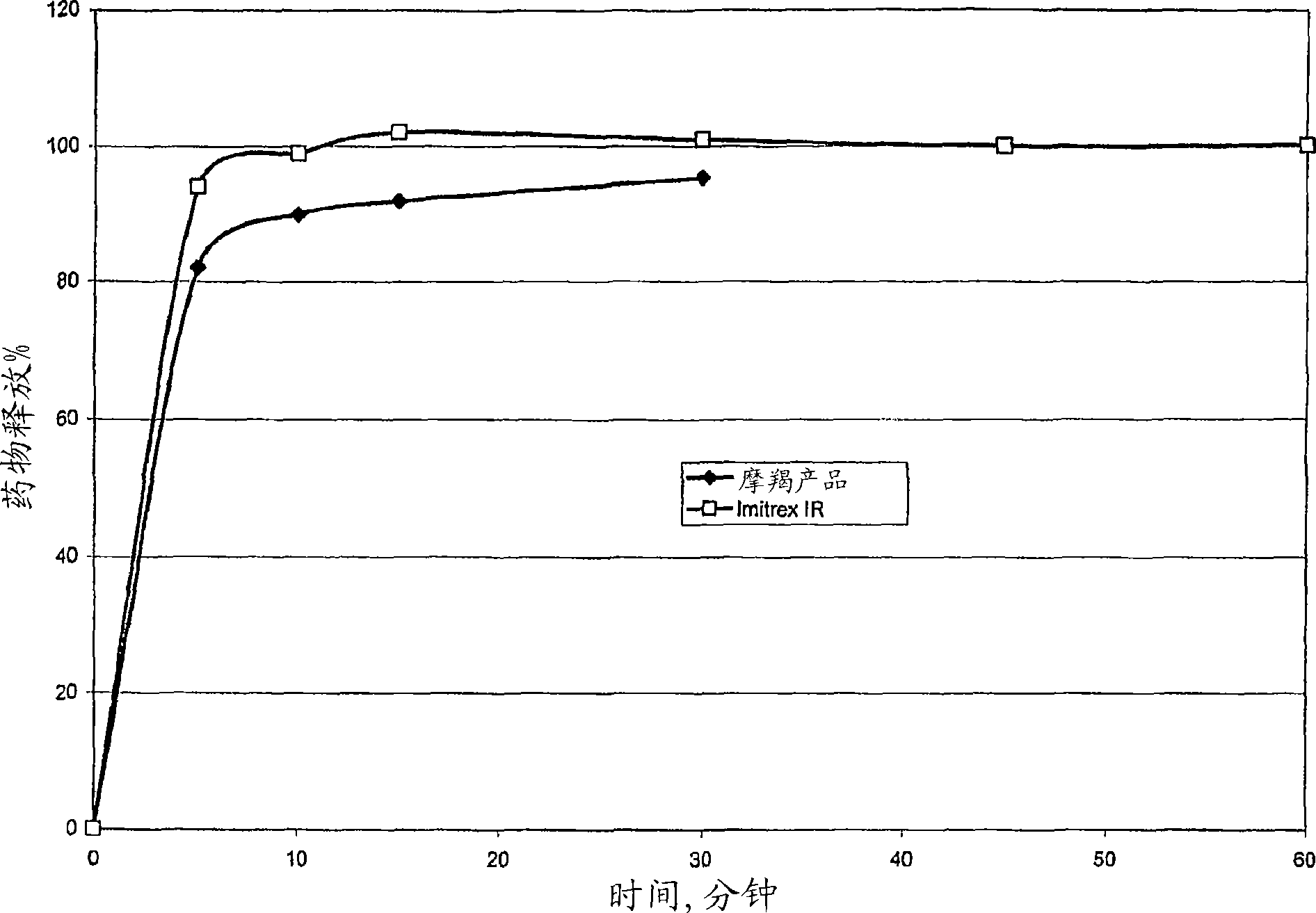
Image
Examples
Embodiment 1
[0086] Example 1 - Taste-masked
[0087] Table 1
[0088]
[0089] The components of Table 1 were used. Sumatriptan succinate was weighed and dissolved in purified water. Amberlite IRP 88 was weighed and added to the drug solution and mixed well for about 6 hours. The drug resin suspension is filtered and the wet cake mixture is separated and dried in a suitable drier at 50° C. for more than 8 hours until the moisture content of the mixture is reduced to about 8-12%. The drying can also be achieved by using a fluidized bed dryer for one hour.
[0090] Similarly, naratriptan, eletriptan, frovatriptan compositions were prepared.
[0091] Similarly, other resins were used to prepare taste-masked triptan compositions. Additional examples were prepared using different coating % resins. These are shown in Table Ia below.
[0092] Table Ia
[0093] Substance name
Embodiment 2
[0094] Example 2 - tableting
[0095] Table 2
[0096] Substance name dry weight% active drug Taste-masked sumatriptan succinate 54.14 excipient Microcrystalline Cellulose Powder 29.28 Lactitol powder 5.00 polyvinylpolypyrrolidone 7.00 Crosslinked Sodium Carboxymethyl Cellulose 1.00 Amberlite IRP 64 2.00 Sodium carbonate 1.00 Magnesium stearate 0.50 glycerin Appropriate amount Artificially Masked Spices Appropriate amount total 100.00
[0097] Tablet 2 was used for compression. Dispersible taste-masking agent powder and other pharmaceutically acceptable ingredients (such as flavors, sweeteners, colors, and additional disintegrants) sufficient to provide a therapeutically effective dose of 25 mg of sumatriptan are placed in a V-blender to fully Practice to obtain a homogeneously distributed mixture for compression. The unit dose is measured on an analytical balance and compressed...
Embodiment 3
[0098] Example 3 - Granulation of mixed sumatriptan
[0099] The blended sumatriptan of Example 2 was granulated using wet granulation methods known in the art. In one aspect, the solvent is an aqueous solvent. Alternatively, solvents such as isopropanol or ethanol may be used. When non-aqueous solvents are used, emulsifiers, fats, waxes, or combinations thereof may be used. These materials can be incorporated via hot melt spraying, or via solution spray granulation systems whereby the solvent is removed. Examples of emulsifiers include acetylated monoglycerides, mono and diglycerides. Waxes can include synthetic and natural waxes and combinations thereof. These microparticles are mixed with other excipients and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com