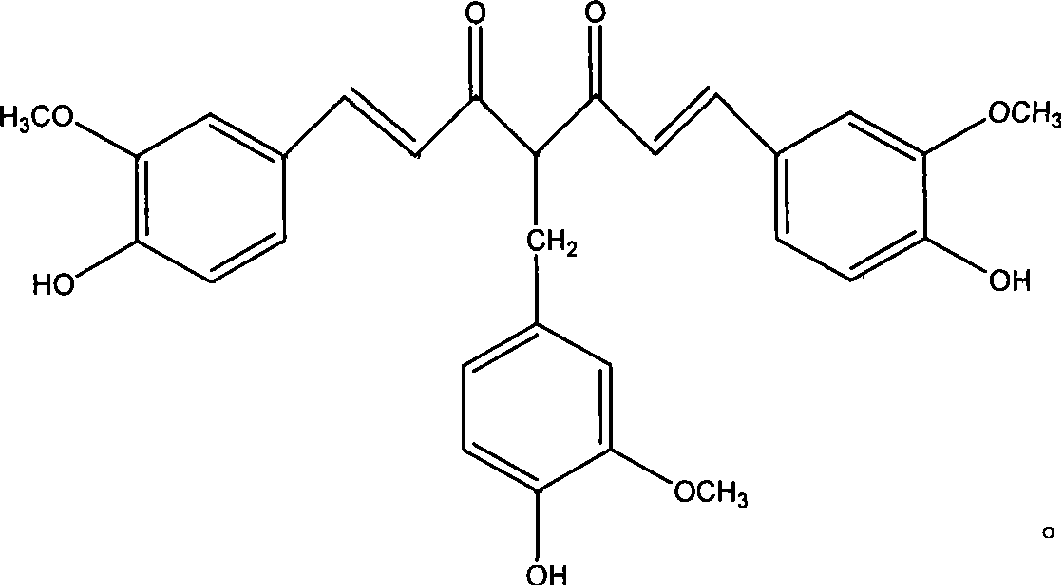
4-(4-hydroxy3-methoxybenzene methyl) curcumin and use thereof in preparing anti-tumor medicament
A technology of methoxybenzyl and methoxybenzylidene, which is applied in the preparation of anti-tumor drugs and the field of synthesizing 4-curcumin, which can solve problems in the in vitro experiment stage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
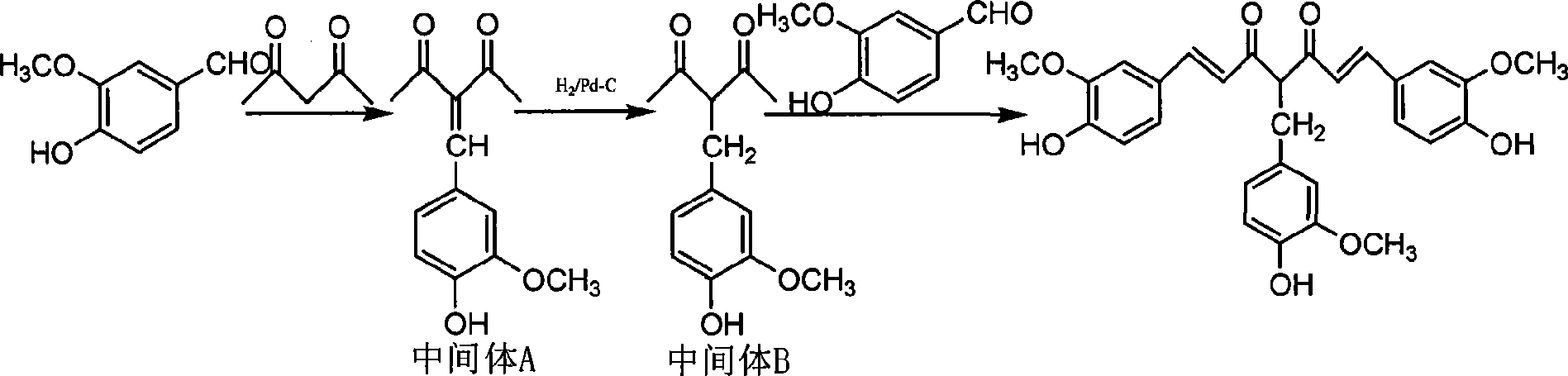
[0012] Example 1 Synthesis of 4-(4-hydroxy-3-methoxybenzyl)curcumin.
[0013] Synthetic raw materials 3-hydroxy-4-methoxybenzaldehyde (vanillin), 2,4-pentanedione, diboron trioxide, tri-n-butyl borate, n-butylamine, and piperidine are all from Sinopharm Chemical Reagent Co., Ltd. company. Catalytic hydrogenation device (Parr 1100, USA), NMR spectrometer (Unity 500, Varian Corporation, USA, 500MHz), ion trap mass spectrometer (DECAX-30000, Thermo Finnigan Corporation, USA); micro melting point apparatus (X-4, Shanghai Precision instrument factory).
[0014] Add 10g (0.1mol) of 2,4-pentanedione and 100ml of ethanol to a 250ml single-necked flask, add a catalytic amount of piperidine, and 15g (0.1mol) of vanillin, stir and react at room temperature for 48 hours, concentrate to remove the solvent, and recrystallize to obtain Light yellow powder intermediate A12.0g.
[0015] Add 100ml of acetone, 10g of intermediate A, 1g of 10% palladium carbon to a 250ml catalytic hydrogenatio...
Embodiment 2
[0017] Example 2 4-(4-hydroxy-3-methoxybenzyl) curcumin inhibits tumor cell K562, HL-60, B-16, SW480, HepG2, MGC80-3, the in vitro growth activity of SH-SY5Y:
[0018] 2.1. Cell lines
[0019] K562: Human chronic myelogenous leukemia blast cell line
[0020] HL-60: Human acute myeloid leukemia cell line
[0021] B16: mouse melanoma B16 cell line.
[0022] SW480: Human colon carcinoma cells
[0023] HepG2: human liver tumor cells
[0024] MGC80-3: human gastric cancer cells
[0025] SH-SY5Y: Human neuroblastoma cells
[0026] The above cells were all obtained from the Shanghai Cell Bank of the Chinese Academy of Sciences.
[0027] 2.2 Cell culture
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap