Conjugates of aziridinyl-epothilone analogs and pharmaceutical compositions comprising same
A heterocycloalkyl compound technology, applied in the field of aziridinyl-epothilone analog conjugates and pharmaceutical compositions containing them, can solve the problem of attacking normal tissues, uneven distribution of antigens, and host toxicity drop and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0083] As noted above, the present invention includes compounds having the following formula I:
[0084]
[0085] And include its pharmaceutically acceptable salts and / or solvates.
[0086] According to one embodiment of the present invention,
[0087] K is O;
[0088] A to C 2-4 alkylene;
[0089] B 1 is -OH;
[0090] R 2 , R 3 , R 4 and R 5 are independently hydrogen or lower alkyl;
[0091] R 6 is hydrogen or methyl;
[0092] R 13 is an optionally substituted 5- or 6-membered heteroaryl, preferably an optionally substituted thiazolyl, pyridyl or oxazolyl; and
[0093] M for -S-R 30 -O-C(=O)-, -S-R 30 -C(=O)-or-S-R 34 R 30 -O-C(=O)-, where
[0094] R 30 is lower alkylene or substituted lower alkylene; and R 34 is arylene or substituted arylene; and R 1 , R 12 , T and Q are as described elsewhere herein, for example in the Summary section above or in the Alternative Embodiments section below.
[0095] One embodiment of the invention provides compounds ...
Embodiment 1
[0232] Example 1: Folic Acid Conjugates Epothilone Analogs
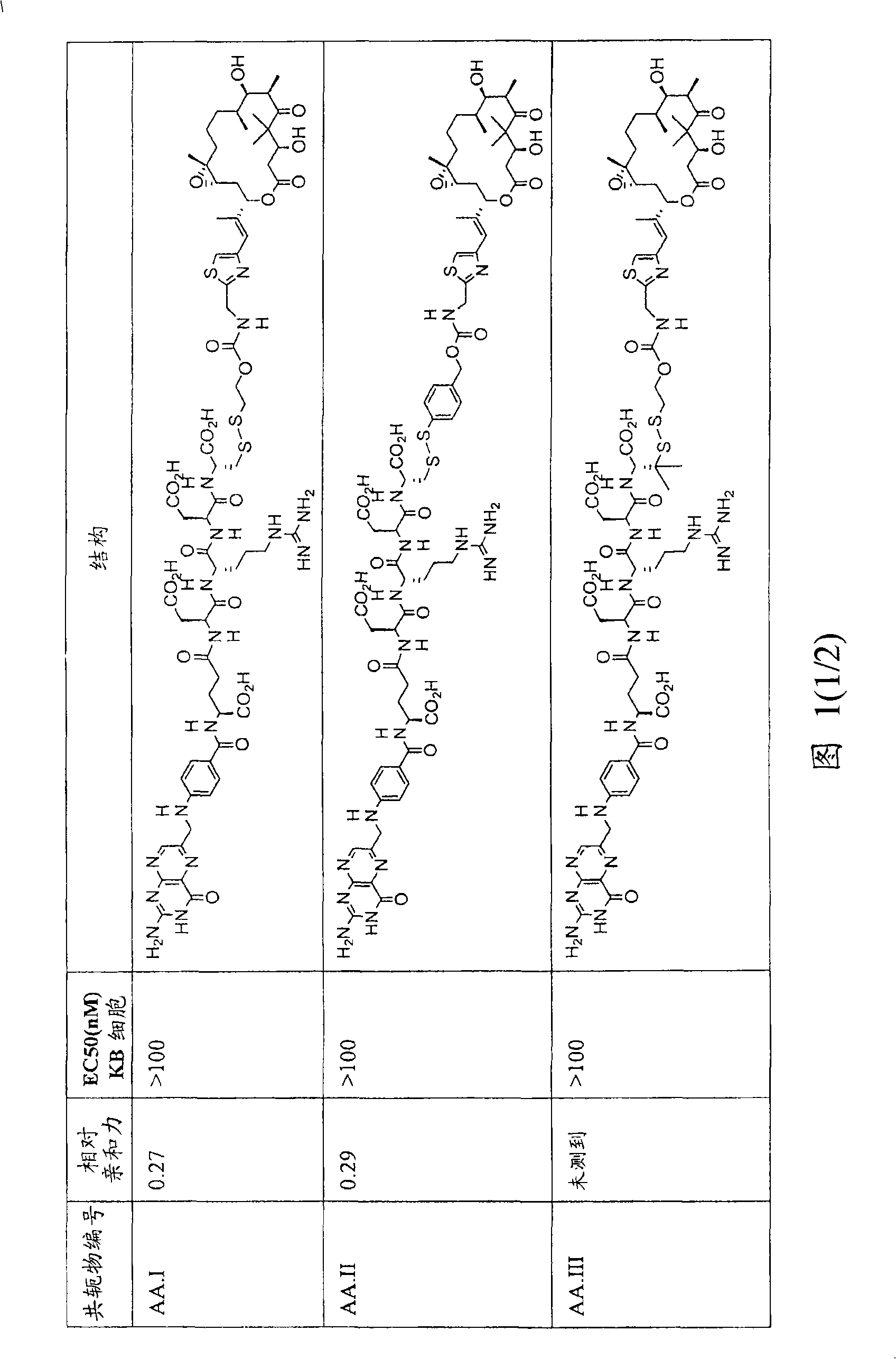
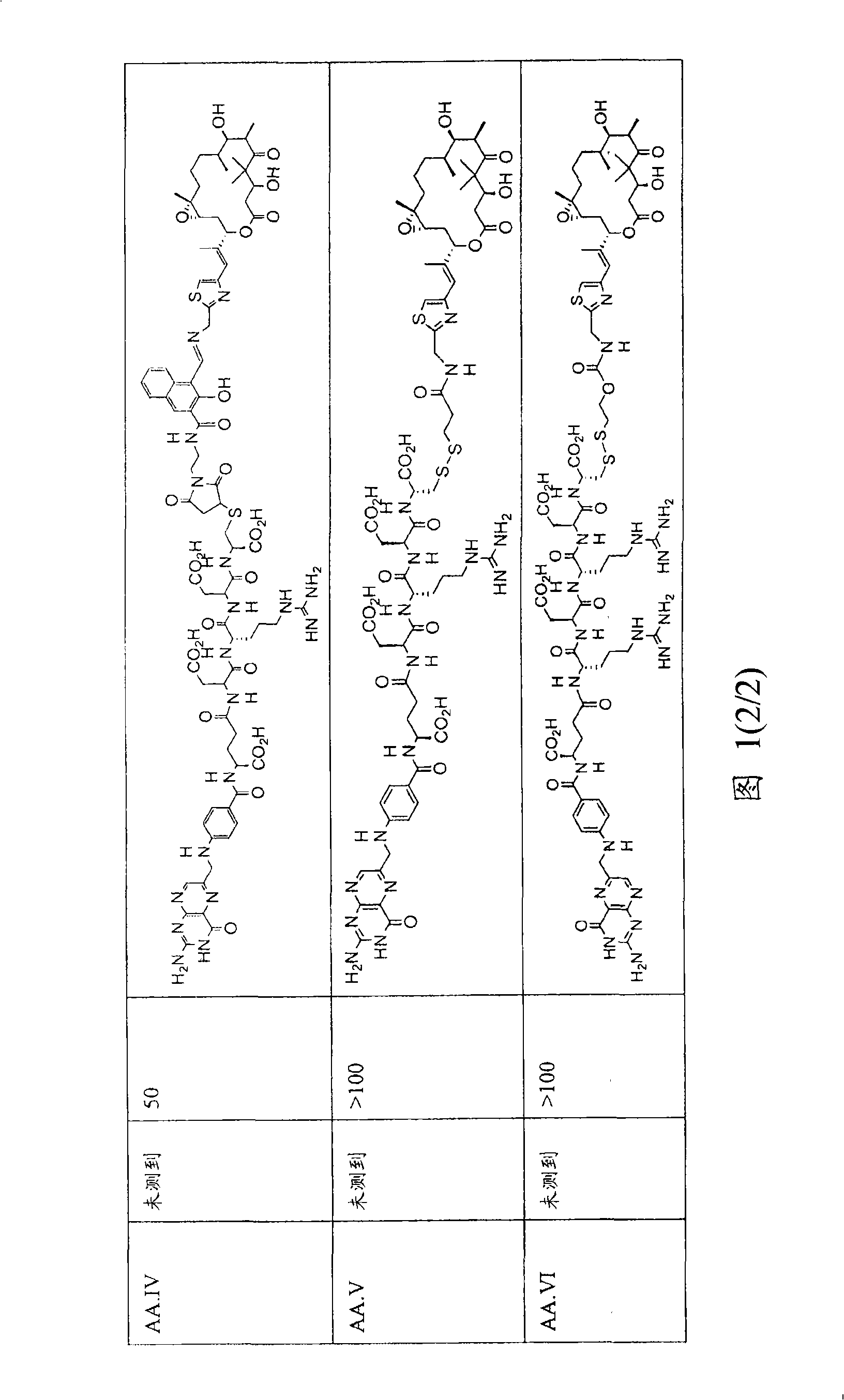
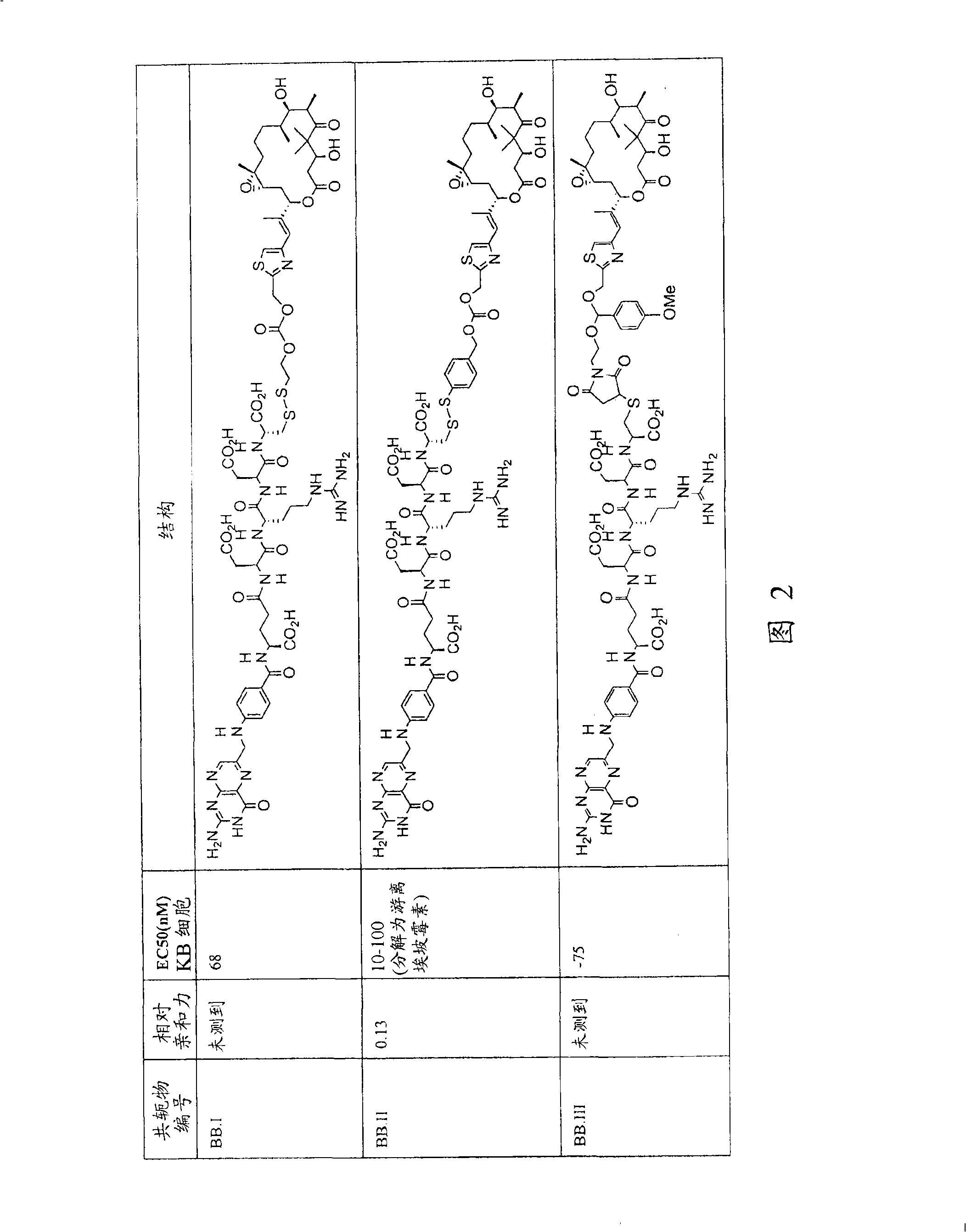
[0233] As noted above in the Summary of the Invention, analogs and derivatives of folic acid are described in Vlahov. In research and development for folate receptor targeting tumor cells conjugated to epothilone and epothilone analog compounds, several compounds were conjugated to folic acid. For example, Compound AA and Compound BB are considered candidates for conjugation with folic acid.
[0234]
[0235] Compound AA Compound BB
[0236] Compound AA was active in Phase II clinical trials, and six folate moiety conjugates of Compound AA (Compounds AA.I to AA.VI) were prepared (see Figure 1) and their chemistry was optionally tested in cell culture. Stability, FR binding and FR-mediated activity.
[0237] Binding of the folate group conjugate of compound AA to FR was determined in an assay measuring the extent of displacement of radiolabeled folate from FR expressed on KB tumor cells grown to confluence. Bindi...
Embodiment 2
[0258] Embodiment 2: preparation compound J
[0259]
[0260] (S)-2-(4-((2-Amino-4-oxo-3,4-dihydropteridin-6-yl)methylamino)benzamido)-5-((S)- 3-carboxy-1-((S)-1-((S)-3-carboxy-1-((R)-1-carboxy-2-(2-(2-((2-((1S,3S , 7S, 10R, 11S, 12S, 16R)-7,11-dihydroxy-8,8,10,12-tetramethyl-3-((E)-1-(2-methylthiazol-4-yl )prop-1-en-2-yl)-5,9-dioxo-4-oxa-17-aza-bicyclo[14.1.0]heptadecan-17-yl)ethoxy)carbonyl Oxy)ethyl)dithio)ethylamino)-1-oxopropan-2-ylamino)-5-guanidino-1-oxopentane-2-ylamino)-1-oxopropane -2-ylamino)-5-oxopentanoic acid
[0261] A.[1S-[1R*, 3R*(E), 7R*, 10S*, 11R*, 12R*, 16S*]]-8,8,10,12-Tetramethyl-3-[1-methanol Base-2-(2-methyl-thiazol-4-yl)vinyl]-7,11-bis[(triethylsilyl)oxy]-4,17-dioxabicyclo[14.1.0 ] Heptadecane-5,9-dione
[0262]
[0263] Triethylsilyl chloride (15.0 mL, 89.4 mmol) was added to N 2 In a stirred solution of epothilone A (5.0 g, 10.1 mmol), imidazole (3.40 g, 49.9 mmol) and DIPEA (28.5 mL, 163.6 mmol) in anhydrous DMF (100 mL) under atmosphe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com