Epothilone analogue, preparation method as well as medicine composition and application thereof
A technology of epothilone and its analogs is used in the preparation of anti-cancer or auxiliary anti-cancer drugs, and the field of pharmaceutical compositions for treating cancer or adjuvant tumor treatment, achieving fewer synthesis steps, wide anti-cancer activity, and large convenience The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
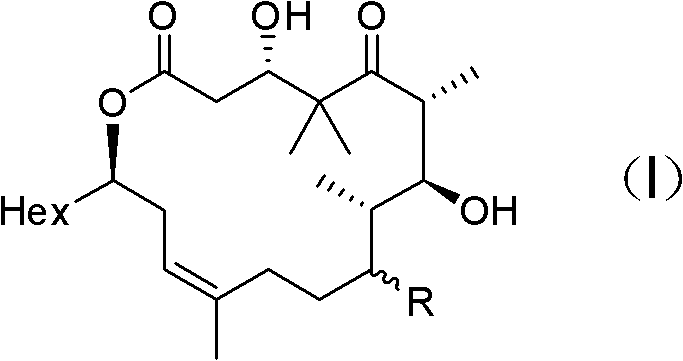
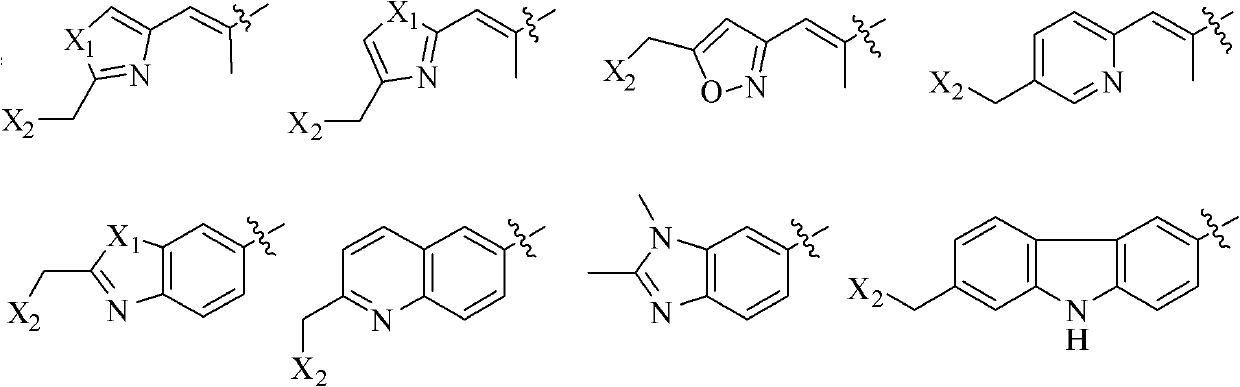
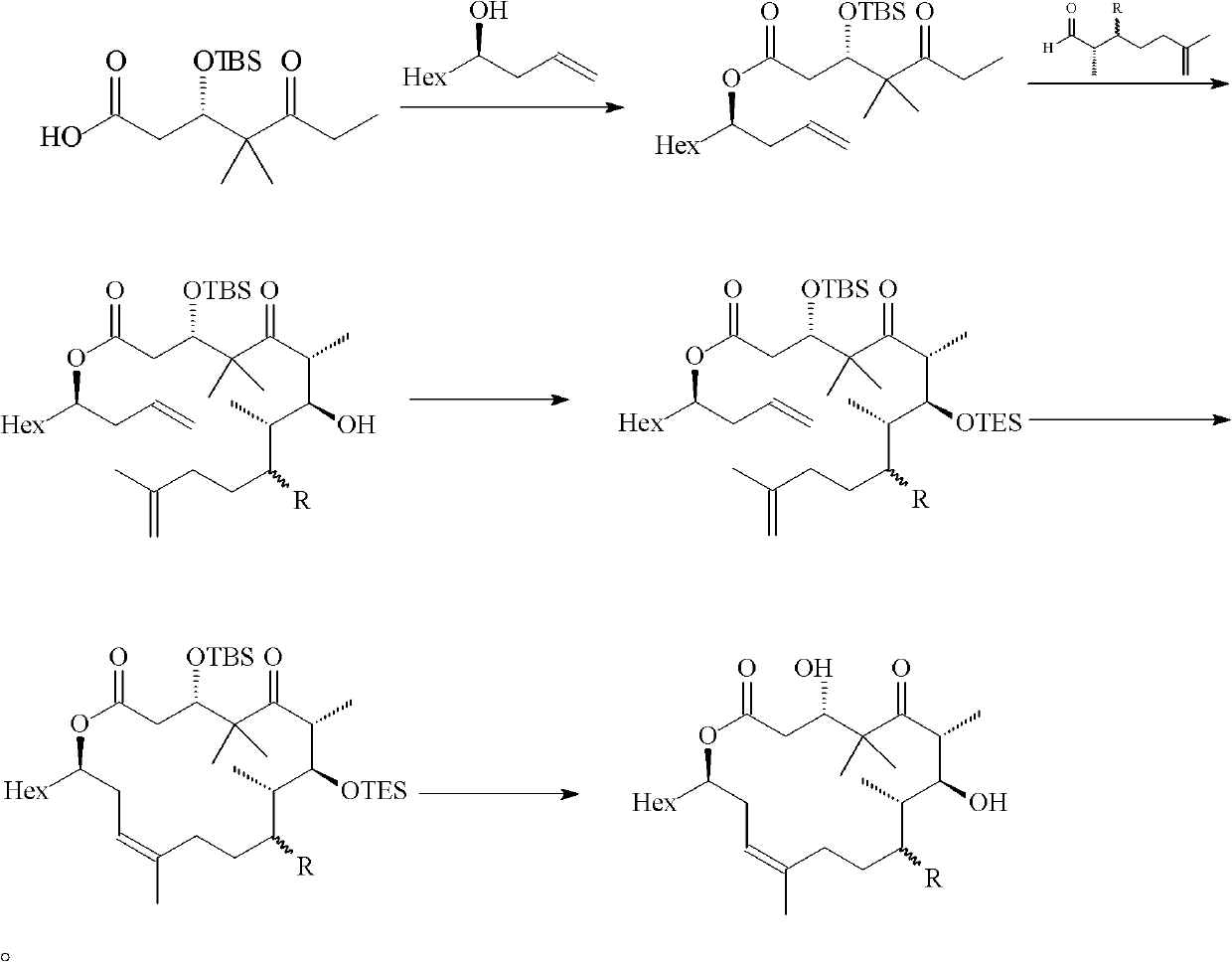
[0047] Embodiment 1: the preparation of formula (III) compound
[0048] Synthesis of compound 3
[0049] Under nitrogen protection, 378mg of compound 1 (1.25mmol), 914mg of compound 2 (4.37mmol) and 7.0mL of dichloromethane were added to a 25mL round bottom flask, and then 306mg of N,N-lutidine (2.5mmol) was added and 959mg 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (5.0mmol), stirred at room temperature for 2h, after the reaction was complete, diethyl ether and water were added to dilute, the aqueous phase was extracted with diethyl ether, and the combined The organic phase was dried with anhydrous magnesium sulfate, filtered with suction, spin-dried to dry the solvent, and purified by column to obtain 470 mg of colorless liquid, yield: 76.3%.
[0050] [α] 20 D =-28.2 (c=10 mg / mL, CHCl 3 ); IR (KBr / cm -1 ): 2954, 2930, 2856, 1737, 1705, 1643, 1505, 1471, 1374, 1293, 1248, 1177, 1088, 1047, 825, 769; 1 H NMR (400MHz, CDCl 3 )δ6.94(s, 1H), 6.48(s, 1H), 5.71(m, 1H), 5...
Embodiment 2
[0067] Embodiment 2: the pharmacological action of epothilone analog
[0068] Take each test cancer cell and make the cells into 2×10 5 / mL cell suspension, add to the 24-well round-bottomed cell culture plate, add the compound of formula (III) respectively, 5 wells for each test concentration, place at 37°C, 5% CO 2 Cultivate under saturated humidity conditions for 48 hours, measure the absorbance (A) value at 570nm wavelength of the enzyme-linked detector by MTT method, and calculate the inhibitory effect of the compound of the present invention on the test cancer cells.
[0069] Table 1 Inhibitory activity of epothilone analogues on cancer cells
[0070]
Compound of formula (III) (IC 50 , μM)
9KB
2.3
4.5
SGC-7901
8.9
MCF-7
1.3
[0071] PC-3
1.1
SK-RC-42
2.5
A549
9.7
HT-29
7.1
K562
5.5
[0072] Among them, 9KB, Hela, SGC-7901, M...
Embodiment 3
[0074] Embodiment 3: injection
[0075] After the compound of formula (III) prepared in Example 1 was dissolved with a small amount of DMSO, water for injection was added as usual, finely filtered, potted and sterilized to make an injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com