Tricyclic compounds
Inactive Publication Date: 2010-09-01
MERCK & CO INC
View PDF6 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Gawley reported that α-aminoorganolithium anions generated from chiral stannane precursors are conformationally stable, although in some cases alkylation with primary alkyl halides yields with excellent enantiomeric selective product, but the method is affected by the fact that the chiral tin precursor must be decomposed
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation Embodiment A
preparation Embodiment B
Embodiment 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
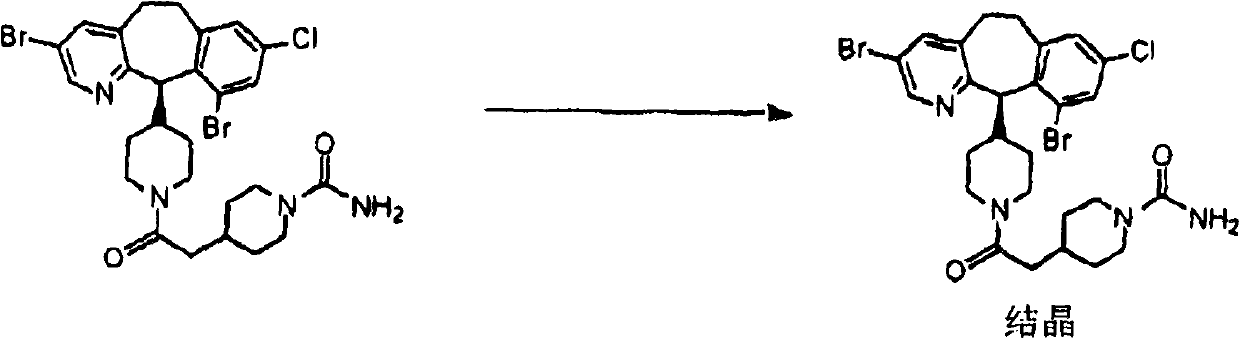
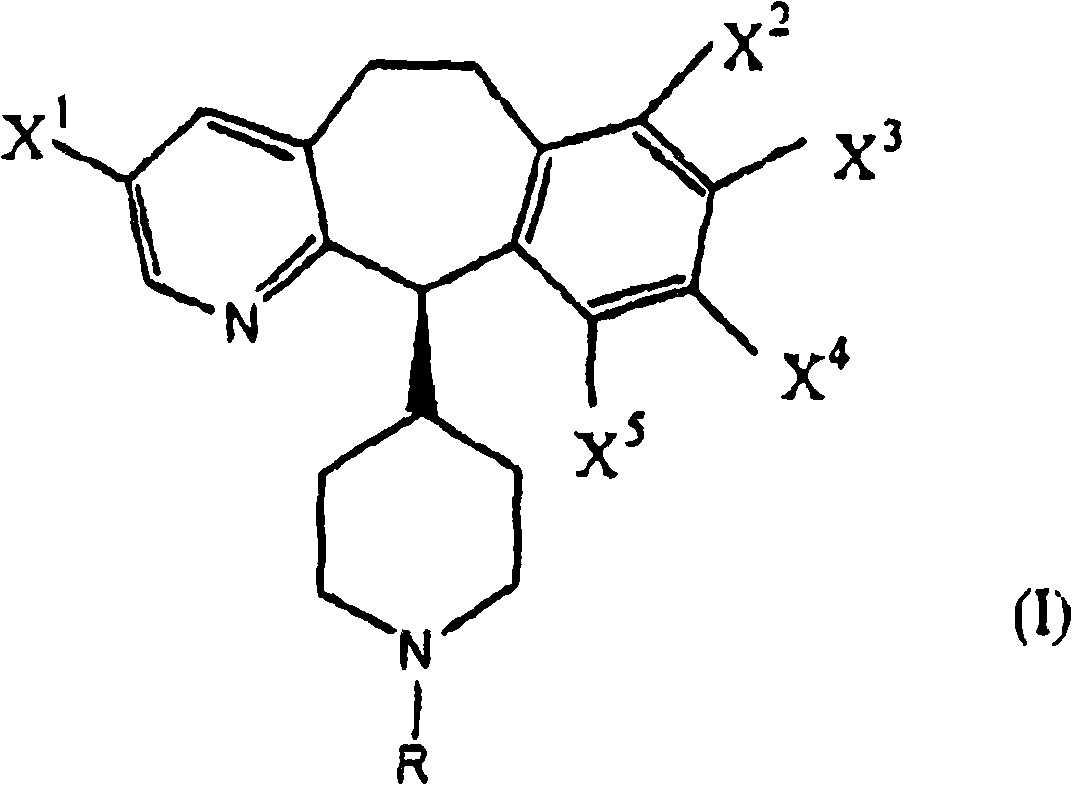
The invention discloses tricyclic compounds of the right formula.
Description
technical field The present invention provides a process for the preparation of intermediates useful in the preparation of known tricyclic compounds which are inhibitors of farnesyl protein transferase. In particular, the compounds prepared by the methods of the present invention are useful as chiral intermediates for the preparation of chiral compounds of FPT inhibitors disclosed in PCT Publication No. WO 97 / 23478 on July 3, 1997. Background technique Over the past few decades, a number of enantioselective carbon-carbon bond-forming reactions have been developed, which can be divided into two distinct classes—one involving the alkylation of covalently bonded chiral precursors and the other Use non-covalent chiral auxiliaries. Examples of the former include Evan's oxazolidinone system, Meyer's oxazoline system and Ender's RAMP / SAMP system. (See Evans, D.A., et al., Encyclopedia of Reagents for Organic Synthesis; Wiley: Chichester, 1995, Vol.1, p.345; Gant, T.G.; Meyers, A....
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D221/16C07B53/00C07B61/00C07D401/04C07D401/14
CPCC07D401/14C07D401/04Y02P20/55
Inventor S·-C·郭C·F·博纳德F·X·陈D·候A·S·金-梅德G·G·吴
Owner MERCK & CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com