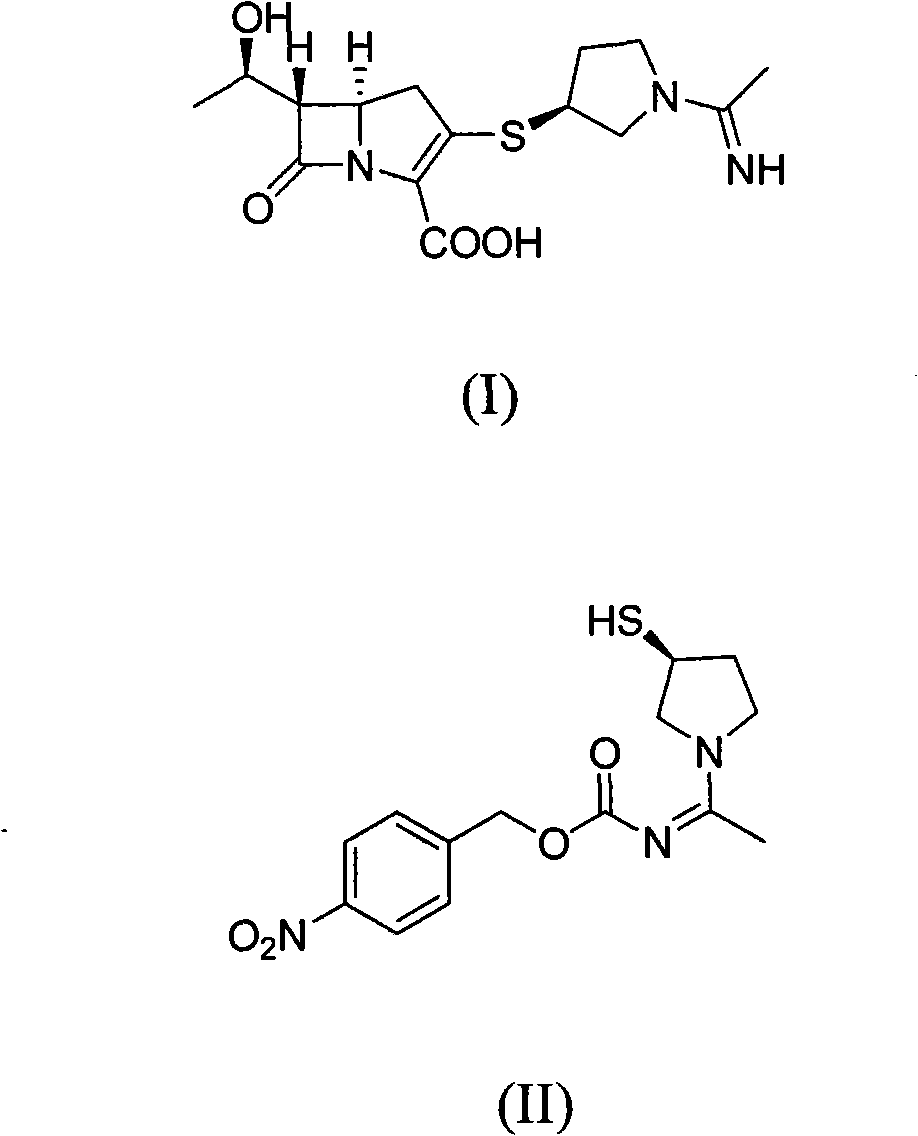
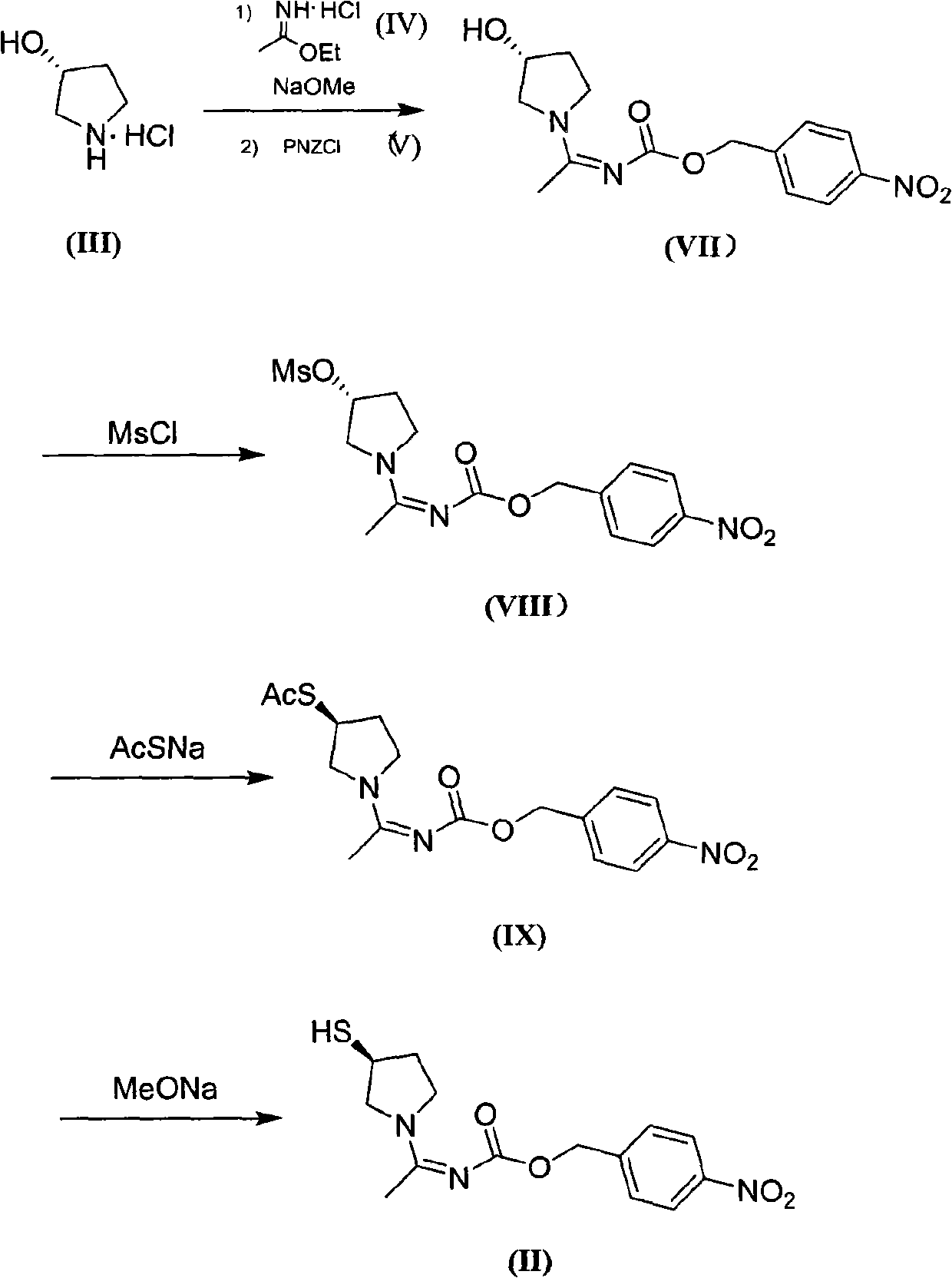
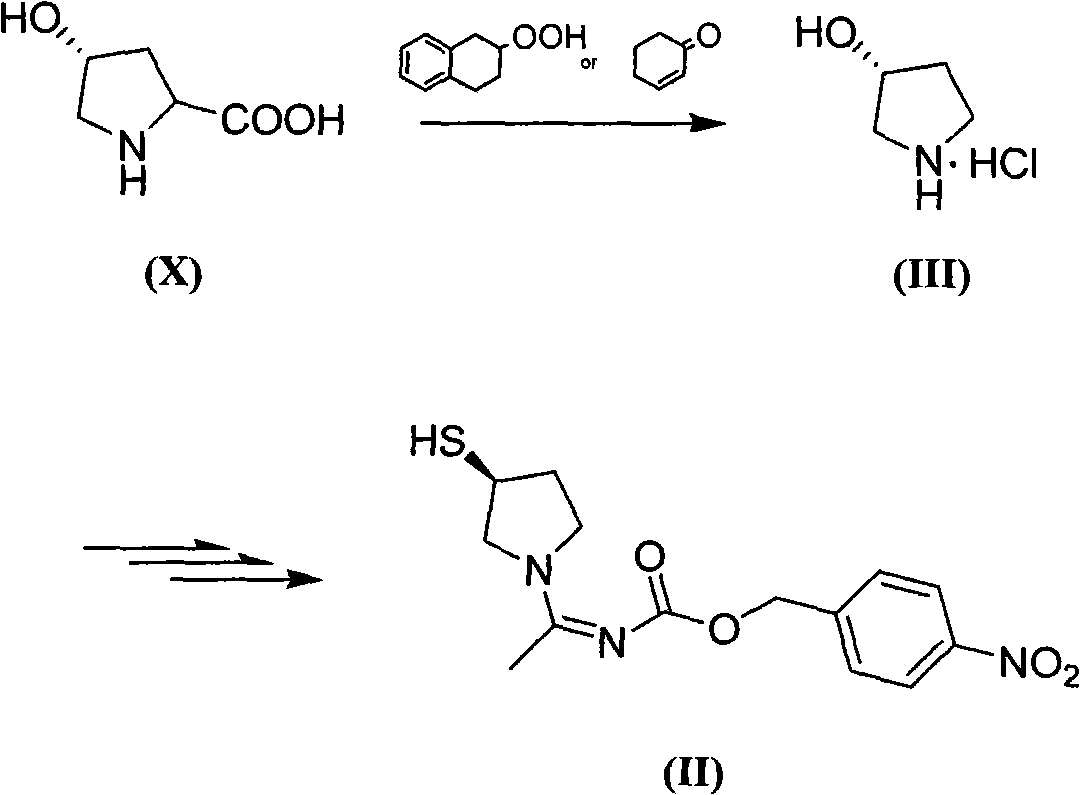
Method for preparing side chain of penipenem
A technology of panipenem side chain and S-3-, which is applied in the field of preparation of panipenem side chain, and can solve problems such as difficulties in post-processing and next step reaction, influence of industrial operation, easy water absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Synthesis of the compound of formula (III)
[0083] 45g of L-4-hydroxyproline (X) and 4g of cyclohexanone-2-ene were added to 250mL of cyclohexanol, the temperature was raised to 140°C, and the reaction was stirred for 3h until the solution became clear. Add dilute hydrochloric acid aqueous solution, stir, stand still, separate liquids, extract three times with 125mL ethyl acetate + 100mL water, combine the water phases, then wash the water layer with 125mL ethyl acetate, add 3.75g activated carbon to the water layer, and bathe for 50 Part of the water was evaporated at ℃, filtered with suction to obtain 700g of an aqueous solution containing about 35g of pyrrolidine hydrochloride, which was placed in the refrigerator and used directly for the next reaction.
Embodiment 2
[0085] Synthesis of the compound of formula (III)
[0086] Add 20g of L-4-hydroxyproline (X) and 1.5g of cyclohexanone-2-ene to 300mL of n-butanol, raise the temperature to 120°C, and stir for 4h until the solution becomes clear. Add dilute hydrochloric acid aqueous solution, stir, stand still, separate liquid, extract three times with 70mL ethyl acetate + 70mL water, combine water phase, then wash water layer with 70mL ethyl acetate, add 2g activated carbon to water layer, water bath 50℃ Part of the water was evaporated by rotary evaporation, and filtered with suction to obtain 350 g of an aqueous solution containing about 12 g of pyrrolidine hydrochloride, which was placed in a refrigerator and used directly for the next reaction.
Embodiment 3
[0088] Synthesis of compounds of formula (XI)
[0089] In 1L of acetonitrile, add 40g of triethylamine at room temperature; under the protection of nitrogen, add 65g of ethyl ether, stir for 1h, cool down to -10°C, add 95g of acetonitrile solution of p-nitrobenzyl chloroformate dropwise, add about 30-40min Finish. Prepare a solution of 40 g of triethylamine in 100 mL of acetonitrile, and add it dropwise to the above reaction solution within 40-60 min (keep at about -5°C). Filtrate, spin dry the filtrate, add 500mL ethyl acetate and 250mL water, stir and separate the layers, extract the water layer once with 250mL ethyl acetate, combine the EA layers, dry over anhydrous sodium sulfate, filter, spin dry, and vacuum dry for 3h to obtain shallow The yellow intermediate (XI) is about 99g, the molar yield is 84.6% (based on p-nitrobenzyl chloroformate), and the purity is 95%.
[0090] The nuclear magnetic resonance data of (XI):
[0091] 1 HNMR (CD 3 OD): 1.26 (t, 3H), 2.05 (s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com