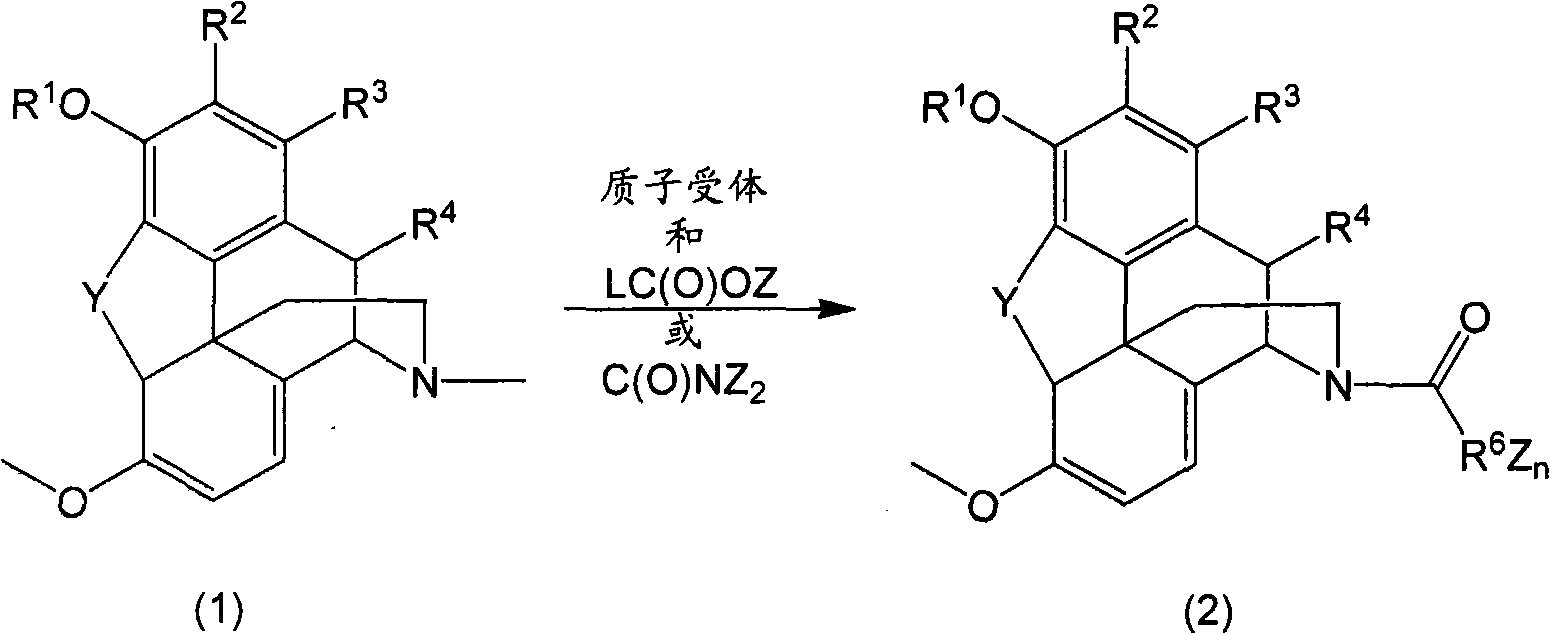
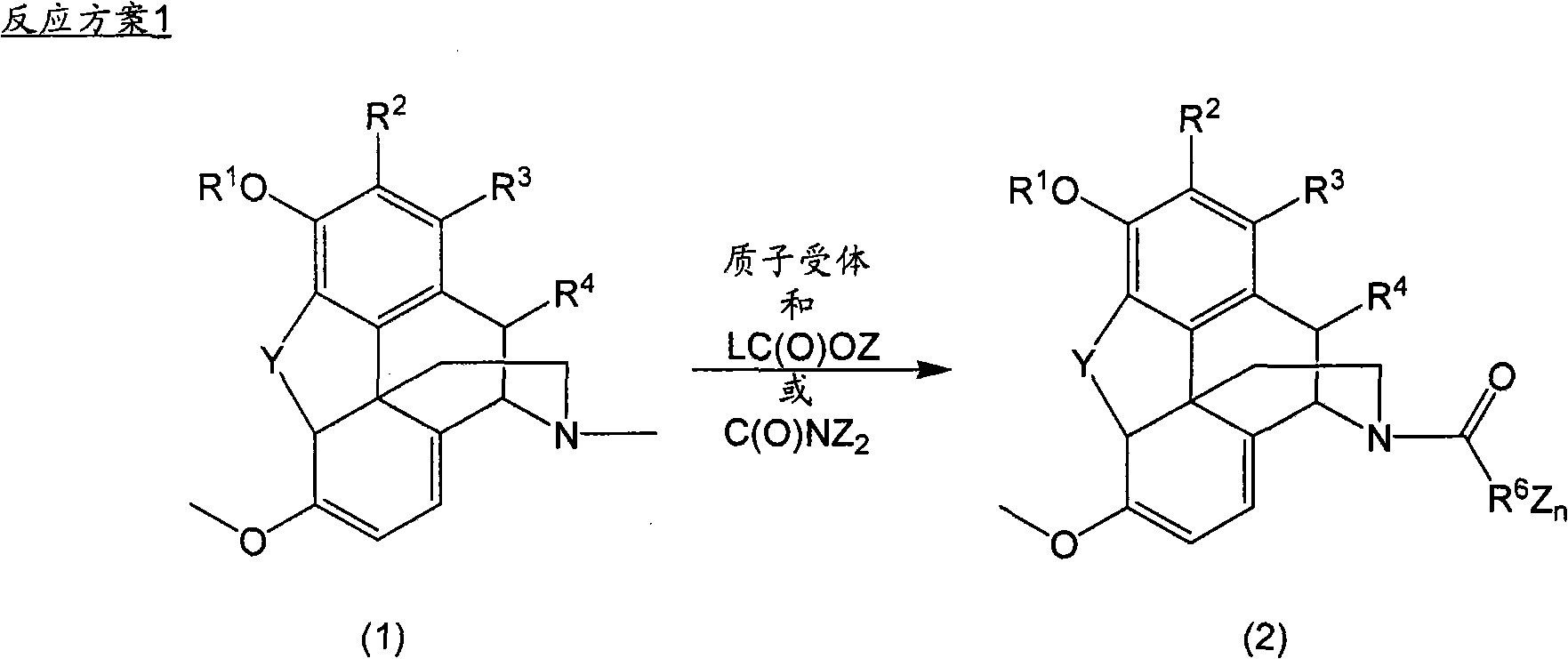
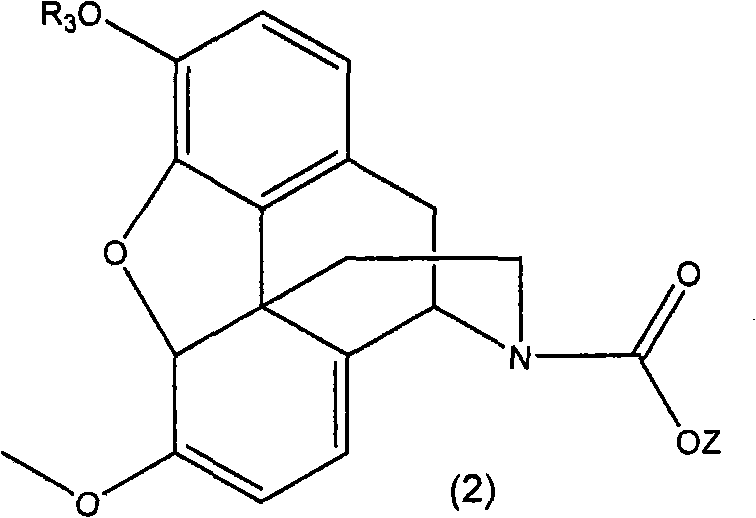
N-demethylation of N-methyl morphinans
A methyl, alkoxycarbonyl technique applied in N-demethylation. field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1. Reaction of oripavine with ethyl chloroformate.
[0136] Oripavine and three different concentrations of ethyl chloroformate (ClCO 2 Et) reaction. Three samples of oripavine were prepared. For each, 0.3 g of oripavine was added to 5 mL of chloroform (CHCl 3 )middle. Once a solution is formed, the mixture is cooled to about 0-5°C. With stirring, 5 mL of saturated NaHCO 3 Add to each mixture. Different amounts of ClCO 2 Et, i.e., 1 equivalent, 2 equivalents or 3 equivalents of ClCO 2 Et (shown in Table 1) was added to each mixture. Each mixture was stirred at room temperature for 30 minutes after the addition of ethyl chloroformate, then sampled for HPLC to determine if the reaction was complete.
[0137] Table 1. Oripavine response.
[0138] sample#
[0139] As shown in Table 1, when oripavine and 3 equivalents of ClCO 2 The reaction was complete when Et (sample #3) reacted. For this sample, the organic solution was 5% NaHCO 3 (9 mL), 5%...
Embodiment 2
[0140] Embodiment 2. The reaction of thebaine and ethyl chloroformate.
[0141] To determine the correct ratio of thebaine and ethyl chloroformate, an experiment similar to Example 1 was carried out. For each sample, 0.31 g of thebaine was dissolved in 5 mL of CHCl 3 middle. Once a solution is formed, the mixture is cooled to about 0-5°C. With stirring, 5 mL of saturated NaHCO 3 Add to each mixture. ClCO 2 Et (as shown in Table 2) was added to each mixture, which was stirred at room temperature for 30 minutes, and then sampled for HPLC to determine whether the reaction was complete.
[0142] Table 2. Thebaine responses.
[0143] sample#
[0144] As shown in Table 2, when thebaine and 3 equivalents of ClCO 2 The reaction was complete when Et (sample #3) reacted. The sample was treated with 5% NaHCO as described above 3 , 5% HOAc and water washes. The organic layer was concentrated to dryness under partial vacuum to give 0.4 g of solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com