Methods and compositions for the treatment of cancer using benzopyrone-type PARP inhibitors
A cancer and compound technology, applied in drug combinations, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., can solve problems such as necrosis, ATP consumption, and cell dysfunction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0578] In Vitro Efficacy of Compound of Formula IIIg (5-iodo-6-nitrocoumarin)
[0579] Materials and methods
[0580] Test Drugs - Cells were plated with vehicle or test drugs at concentrations of 0.1 μM, 0.3 μM, 1 μM, 3 μM, 10 μM, 30 μM and 100 μM and treated for 24 hours (day 1).
[0581] Tumor Cell Lines—Tumor cell lines (Table 1) were obtained from the American Type Cell Collection (ATCC, Rockville, MD) or the NCI-DCTD Tumor Repository (Bethesda, MD) and were stored in media supplemented with 10% fetal bovine serum (FBS, NovaTech). Store it in a specific growth medium. Cells were propagated at 37°C in a humidified atmosphere containing 5% carbon dioxide.
[0582] Cell Proliferation Assay - Cell proliferation was determined using the BrdU chemiluminescent assay, which measures the incorporation of 5-bromo-2'-deoxyuridine (BrdU) into the genomic DNA of proliferating cells. Briefly, cells and BrdU are added for 4 hours, during which time it is incorporated into the DN...
Embodiment 2
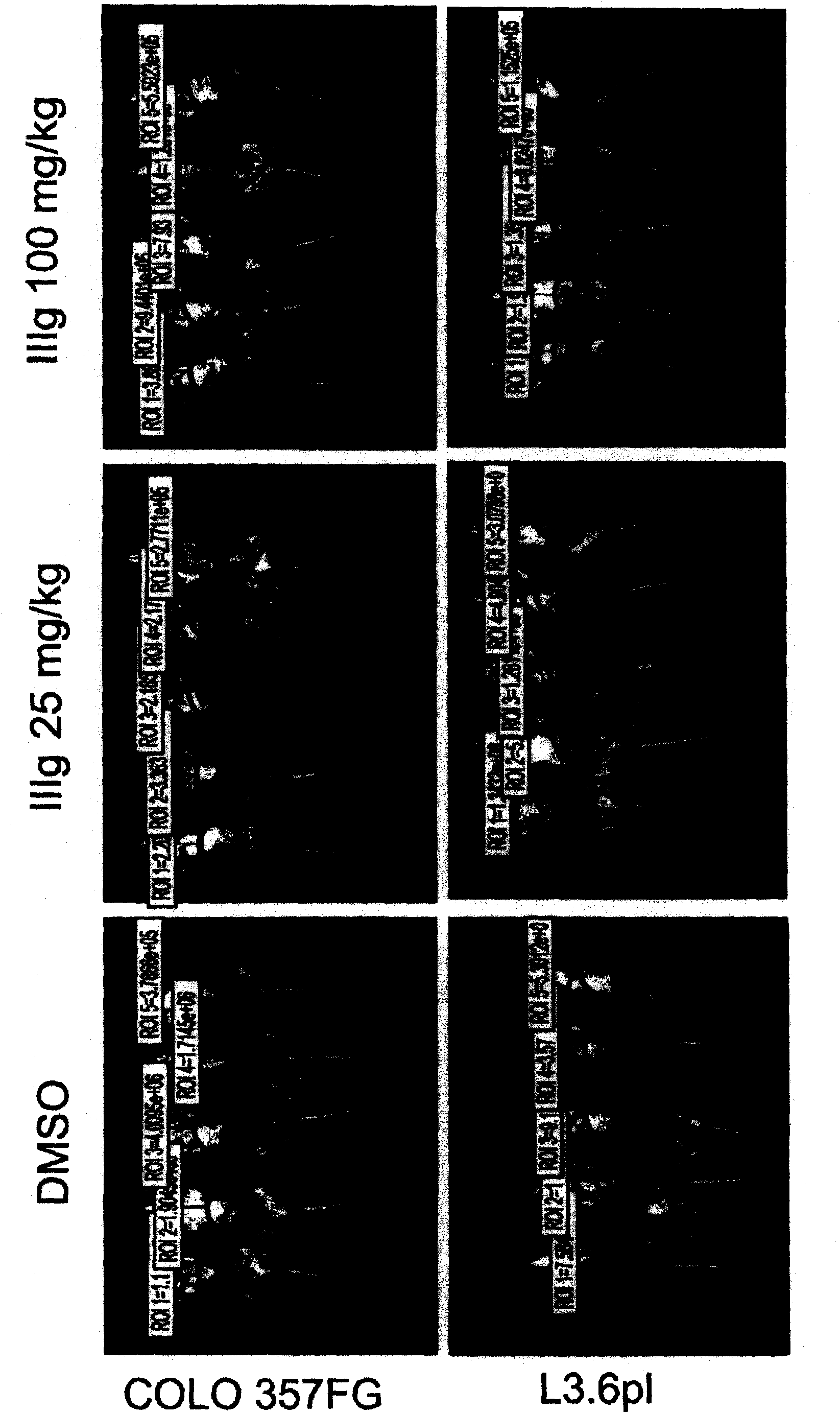
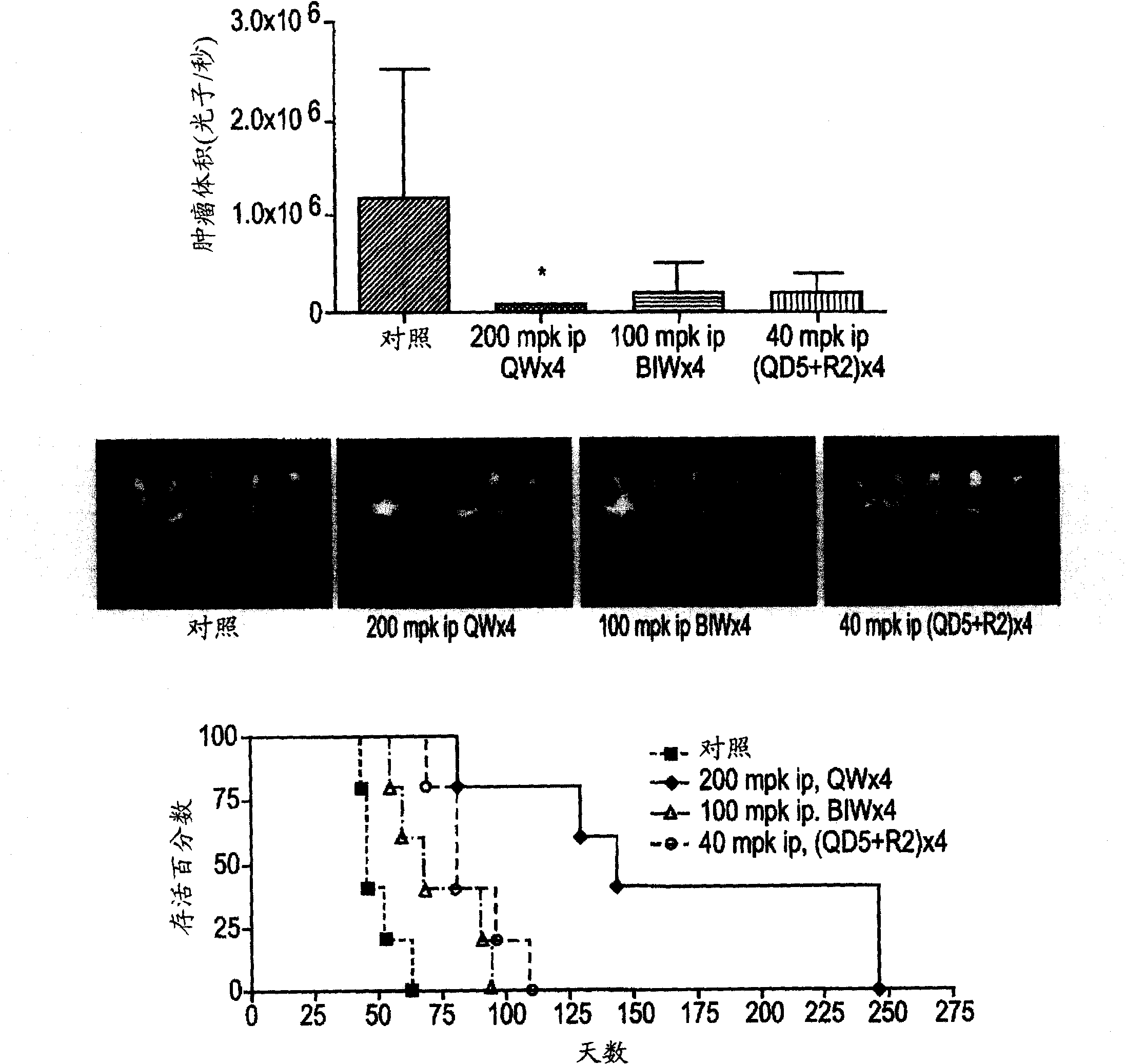
[0602] Example 2: Evaluation of IIIg on human cancer xenograft implants in nude mice.
[0603] Methods and materials
[0604] mouse
[0605]Female athymic nude mice (nu / nu, Harlan) were 9-10 weeks old and had a body weight (BW) range of 18.1-27.0 g on study day 1 . Let animals drink water (reverse osmosis water, 1ppm Cl) freely and eat (NIH 31 Modified and Irradiated Lab Diet Containing 18.0% crude protein, 5.0% crude fat and 5.0% crude fiber). ALPHA-dri irradiated in static microisolation chambers in mice bed-o-cobs The experimental animals were raised on beds, and the lights were turned on for 12 hours, the temperature was 21-22°C (70-72°F), and the humidity was 40-60%. The PRC explicitly follows the recommendations of the "Guide for Care and Use of Laboratory Animals" regarding confinement, husbandry, surgical procedures, dietary adjustments, and veterinary care. PRC's animal program is accredited by AAALAC International to ensure compliance with generally accepted...
Embodiment 3
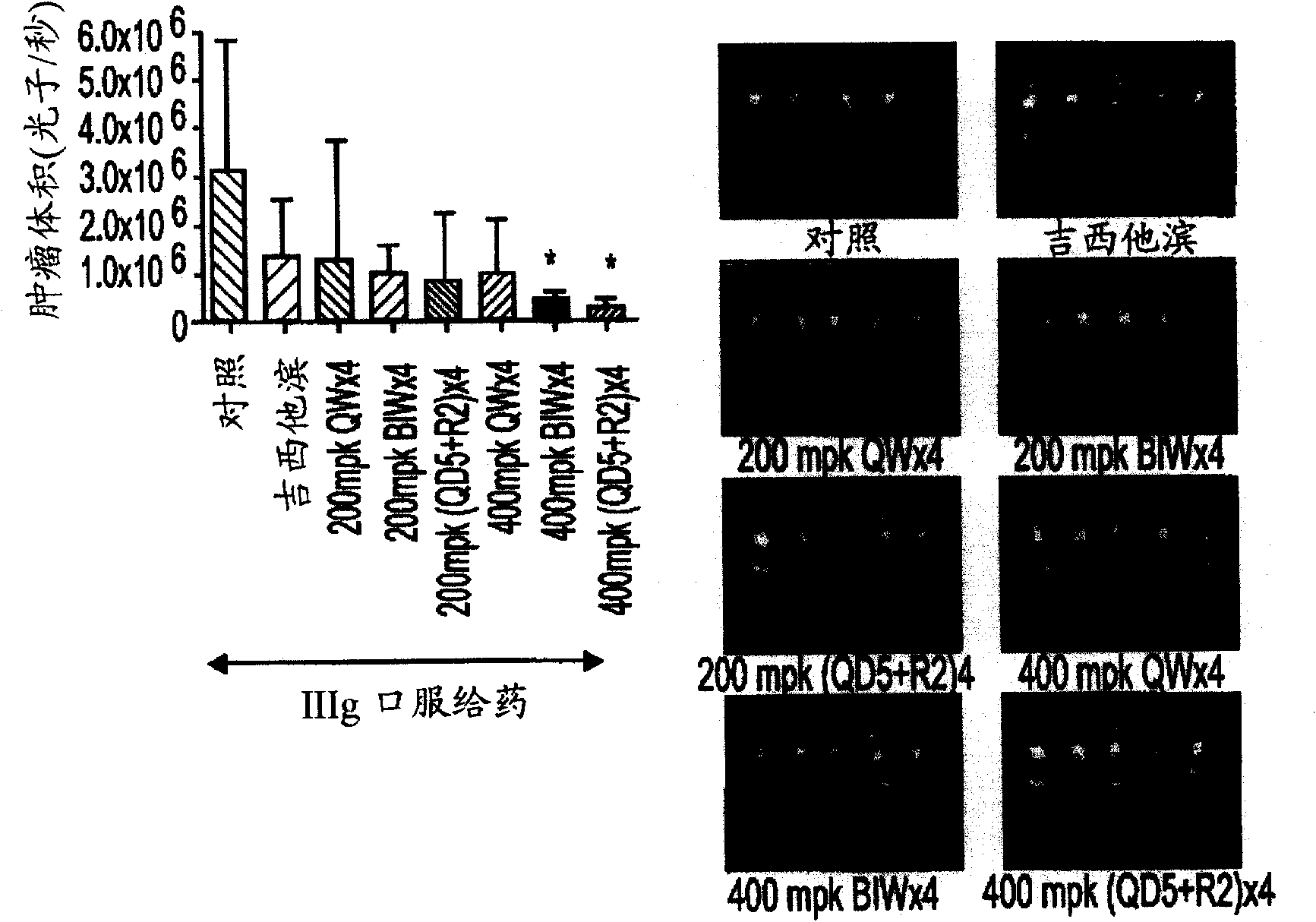
[0649] Example 3: Efficacy of IIIg on pancreatic cancer cell lines
[0650] Pancreatic cancer cell lines and culture conditions
[0651] Human pancreatic cancer cell lines were maintained in minimal essential medium supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, non-essential amino acids, l-glutamine, double vitamin solution (Life Technologies, Grand Island, NY ) and penicillin-streptomycin mixture (Flow Laboratories, Rockville, MD). Cultures were free of mycoplasma and the following pathogenic murine viruses: reovirus type 3, pneumovirus, K virus, Theiler encephalitis virus, Sendai virus, parvovirus, mouse adenovirus, mouse hepatitis virus, lymphocytic choriomeninges Influenza virus, pox virus and lactate dehydrogenase virus (analyzed by Science Applications International Corp. Frederick, MD).
[0652] Orthotopic Transplantation of Animal and Tumor Cells
[0653] Male athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the Natio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com