Synthesis method of benzopyrone and imidazopyridine compound
A technology of imidazopyridine and benzopyrone, which is applied in the field of synthesis of benzopyrone imidazopyridine compounds, can solve the problems of compound synthesis methods and reports, and avoid waste of resources and environmental pollution , high reaction efficiency and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
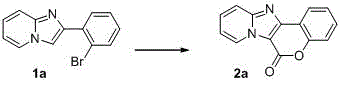
[0020] In a 15mL Schlenk tube add 1a (0.5mmol, 136.6mg), DMSO (2mL), tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 0.025mmol, 23mg), Cu(OAc) 2 (0.25mmol, 45.4mg), triphenylphosphine (0.075mmol, 20mg) and 1,4-diazabicyclo[2.2.2]octane (DABCO, 1.5mmol, 168.3mg). Evacuate and fill with CO, repeat three times. Stir the reaction for 12 hours in an oil bath at 150° C. under a CO atmosphere of 1 atm. The reaction was quenched by adding 10 mL of saturated ammonium chloride solution, extracted with ethyl acetate (6 mL×3), and then the organic phase was washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. Filter, spin dry, and separate through silica gel column (petroleum ether / ethyl acetate=2 / 1) to obtain the target product 2a (21.2mg, 18%). The characterization data of this compound are as follows: 1 HNMR (400MHz, CDCl 3 )δ:7.19(t, J =7.2Hz,1H),7.43(t, J =7.6Hz,1H),7.50(d, J =8.4Hz,1H),7.55-7.60(m,1H),7.63-7.67(...
Embodiment 2
[0022] In a 15mL Schlenk tube add 1a (0.5mmol, 136.6mg), DMSO (2mL), Pd2 (dba) 3 (0.025mmol, 23mg), Cu(OAc) 2 (0.5mmol, 90.8mg), triphenylphosphine (0.075mmol, 20mg) and DABCO (1.5mmol, 168.3mg). Evacuate and fill with CO, repeat three times. Stir the reaction for 12 hours in an oil bath at 150° C. under a CO atmosphere of 1 atm. The reaction was quenched by adding 10 mL of saturated ammonium chloride solution, extracted with ethyl acetate (6 mL×3), and then the organic phase was washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. Filter, spin dry, and separate through silica gel column (petroleum ether / ethyl acetate=2 / 1) to obtain the target product 2a (35.4mg, 30%).
Embodiment 3
[0024] In a 15mL Schlenk tube add 1a (0.5mmol, 136.6mg), DMSO (2mL), Pd 2 (dba) 3 (0.025mmol, 23mg), Cu(OAc) 2 (1.0mmol, 181.6mg), triphenylphosphine (0.075mmol, 20mg) and DABCO (1.5mmol, 168.3mg). Evacuate and fill with CO, repeat three times. Stir the reaction for 12 hours in an oil bath at 150° C. under a CO atmosphere of 1 atm. The reaction was quenched by adding 10 mL of saturated ammonium chloride solution, extracted with ethyl acetate (6 mL×3), and then the organic phase was washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. Filter, spin dry, and separate through silica gel column (petroleum ether / ethyl acetate=2 / 1) to obtain the target product 2a (24.8mg, 21%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com