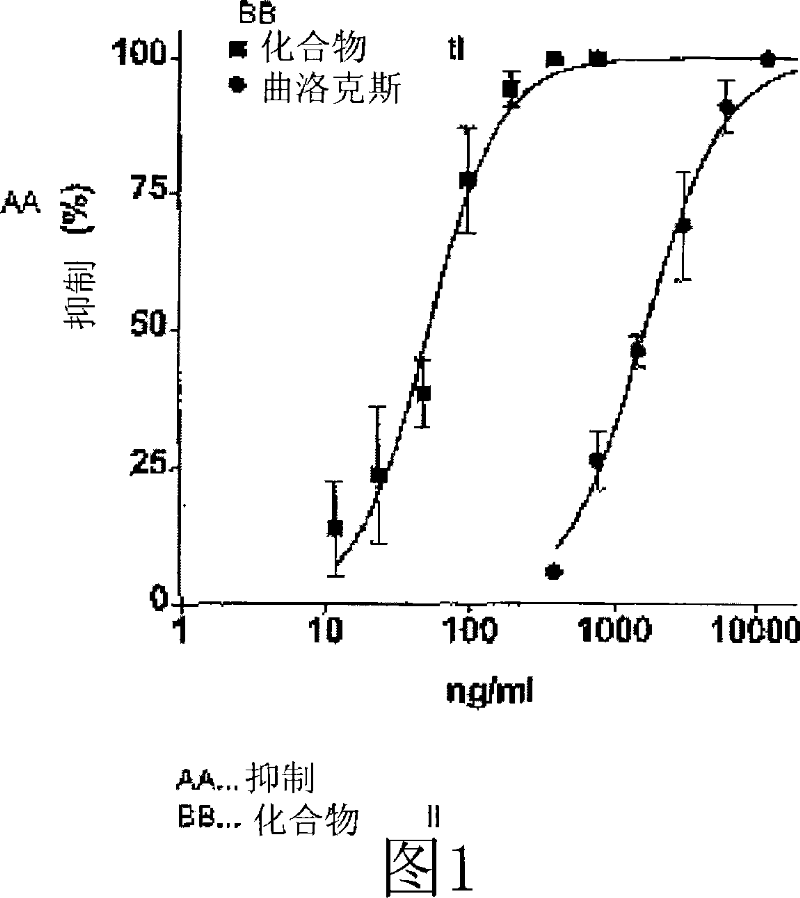
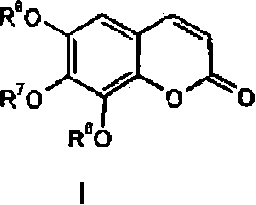
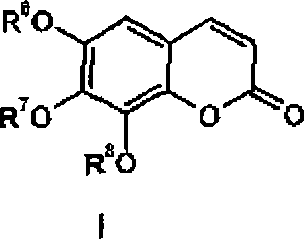
Use of trisubstituted benzopyranones
A technology of use and extract, applied in the field of tri-substituted benzopyrones, can solve the problems of continuous formation of harmful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] Example 1: Preparation of 6,7,8-trihydroxy-2H-1-benzopyran-2-one (compound II)
[0039] At 40 to 50°C, 20g (42.7mmol) of 6,8-bis(sulfoxy)-7-hydroxyl-2H-1-benzopyran-2-one potassium salt was stirred in 480ml of about 2N hydrochloric acid for 20h . After cooling, the precipitated crude product is filtered and recrystallized from water (hot filtration). The crystals were filtered, washed and dried in vacuo at 100°C: 5.9 g (71%), melting point: decomposition started at 260°C; 1 H and 13 CNMR follows the instructions of O. Kayser and H. Kolodziej (Phytochemistry 39, 1181-1135 (1995)).
example 2
[0040] Example 2: Isolation and structure determination of 6,8-bis(sulfoxy)-7-hydroxy-2H-1-benzopyran-2-one potassium salt (potassium salt of compound III)
[0041] 15 kg of ground roots of Pelargonium sidoides were percolated twice with 75 1 and 40 1 of water respectively at room temperature. The aqueous extract was concentrated to about 1 / 3, 7 kg of ammonium sulfate was added thereto and extracted several times with a 3 / 2 mixture of 2-butanone / ethanol. The organic phases were combined and concentrated by evaporation.
[0042] The residue was chromatographed on a HP20 column (eluent: water). The 6,8-bis(sulfoxy)-7-hydroxy-2H-1-chromen-2-one fraction was concentrated, adjusted to pH 8 with potassium hydroxide solution and diluted with ethanol at a ratio of 1 / 1 . The precipitate was filtered and suspended in water. The pH was adjusted to 10.7 with potassium hydroxide solution and diluted with ethanol at a ratio of 1 / 1. The precipitate thus settled was redissolved in hot wa...
example 3
[0049] Example 3: Plant extract with a certain content of 6,8-bis(sulfoxy)-7-hydroxyl-2H-1-benzopyran-2-one (compound III)
[0050] 500 g of ground roots of Pelargonium sidoides were extracted with 3 kg of water over 4 h at room temperature. The extracted plant material was filtered and extracted once more with 2 kg of water and filtered as above. The filtrates were combined, concentrated at about 35°C and lyophilized: 58.7 g (11.7%) of dry extracts, of which the content of compound III was 1.54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com