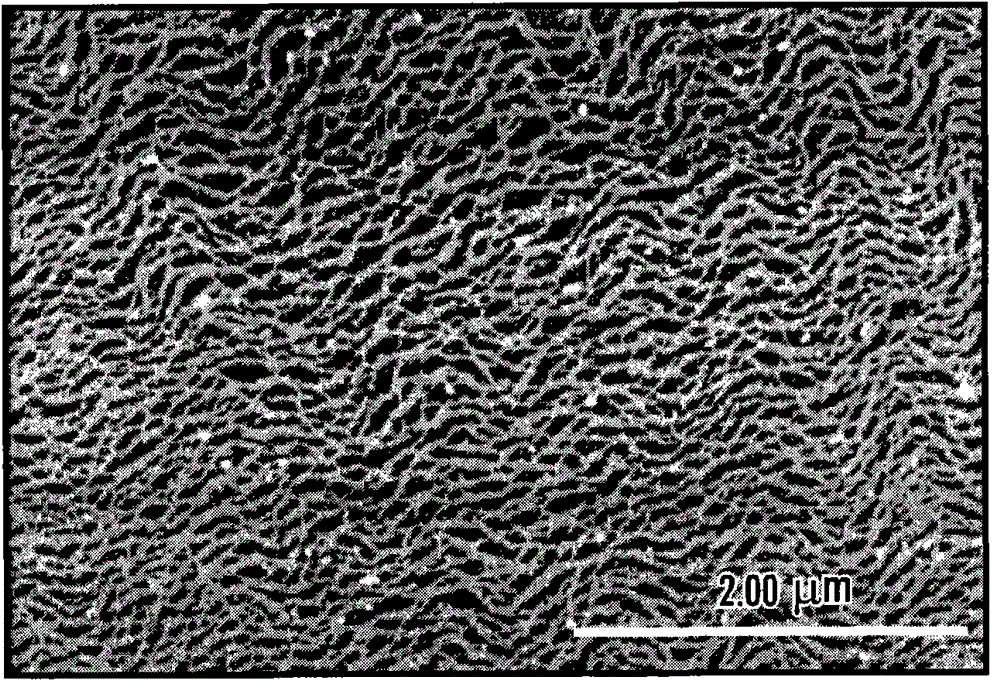
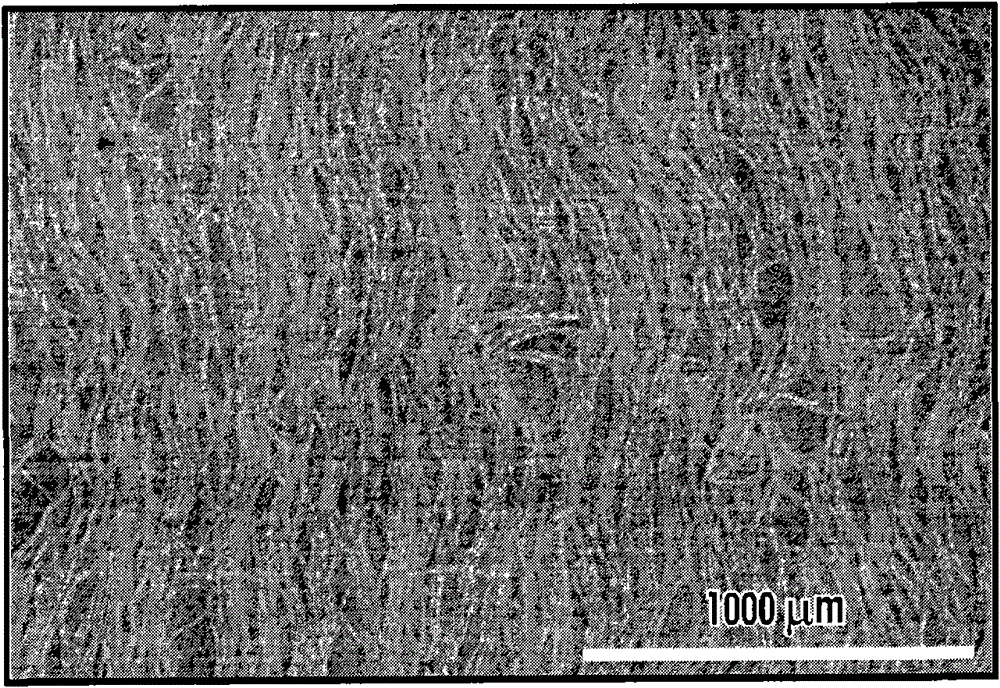
self-assembled nanostructures
A nanostructure, selected technology, applied in the field of forming these self-assembled nanostructures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1 : Benzoic acid derivatives (Table 1, compound 10 (m=11, n=9)):
[0118]
[0119] Step I: Synthesis of 2-decyltetradecanoyl chloride
[0120] Under an inert atmosphere, 2-decyltetradecanoic acid (ISOCARB24, purchased from Sasol America, 1.15 g, 3.13 mmol) and dry tetrahydrofuran (20 mL) were mixed in a 100 mL vessel with stirring. The mixture was cooled to 0 °C for at least 30 minutes, a catalytic amount of N,N'-dimethylformamide (4 drops) was added, followed by slow dropwise addition of oxalyl chloride (1 mL, 12.6 mmol). The reaction was then allowed to warm slowly to room temperature and the reaction was stirred for 30 minutes before the solvent was removed by rotary evaporation. The acid chloride compound thus obtained was used in the next step without further purification.
[0121] Step II: Synthesis of methyl 3,5-bis(2'-decyltetradecylamido)benzoate
[0122] Methyl 3,5-diaminobenzoate (260.8 mg, 1.9 mmol) was dissolved in dry tetrahydrofuran (5 mL)...
Embodiment 2
[0125] Example 2 : 5-(2-decyltetradecylamino)isophthalic acid (Table 1, compound 53):
[0126]
[0127] Step I: Synthesis of 2-decyltetradecanoyl chloride
[0128] Under an inert atmosphere, in a 500 mL single necked round bottom flask was added 2-decyltetradecanoic acid (ISOCARB24, purchased from Sasol America, TX, 7.65 g, 20.8 mmol) and dry tetrahydrofuran (100 mL). A catalytic amount of N,N'-dimethylformamide (0.28 mL, 3.64 mmol) was then added followed by slow dropwise addition of oxalyl chloride (7.3 mL, 83.7 mmol). The mixture was stirred for 10 minutes until the evolution of hydrogen chloride gas ceased. The mixture was then stirred for an additional 3 hours before the solvent was removed by rotary evaporation to obtain a thick pale yellow syrup. The acid chloride compound thus obtained was used in the next step without further purification.
[0129] Step II: Synthesis of dimethyl 5-(2'-decyltetradecylamido)isophthalate
[0130] Dimethyl 5-aminoisophthalate (Al...
Embodiment 3
[0133] Example 3 : 5-(2-decyltetradecylamido)isophthalamide (Table 1, compound 74 (m=11, n=9)):
[0134]
[0135] With stirring under an inert atmosphere, to a 100 mL round bottom flask was added 0.50 g of 5-(2′-decyltetradecylamido)isophthalic acid (from Example 2, 0.89 mmol) dissolved in 20 mL of anhydrous tetrahydrofuran ). 0.3 mL of oxalyl chloride (3.55 mmol) and 2 drops of N,N'-dimethylformamide were added and the reaction was stirred for 2 hours before the tetrahydrofuran was removed by rotary evaporation. The crude solution was resuspended in 5 mL of dry tetrahydrofuran under nitrogen, then cooled to 0-5 °C using an ice-water bath. Then 4 mL of concentrated 30% ammonium hydroxide was added and the reaction was allowed to slowly warm to room temperature and stir for 2 days. Solvent was removed by rotary evaporation. The crude solid was then resuspended in 75 mL of chloroform and washed with 50 mL of deionized water. Chloroform was removed by rotary evaporation....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com