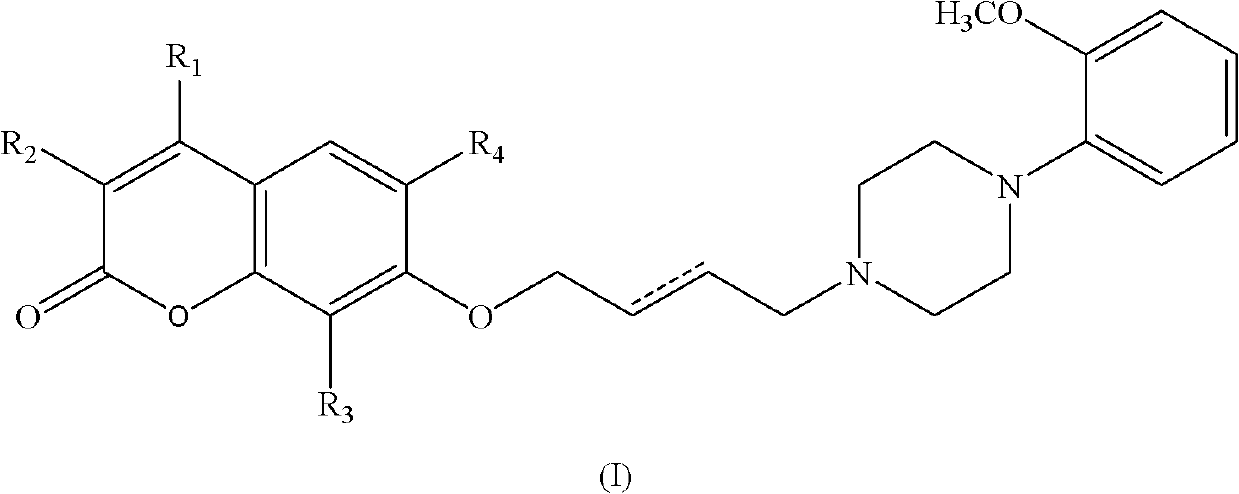
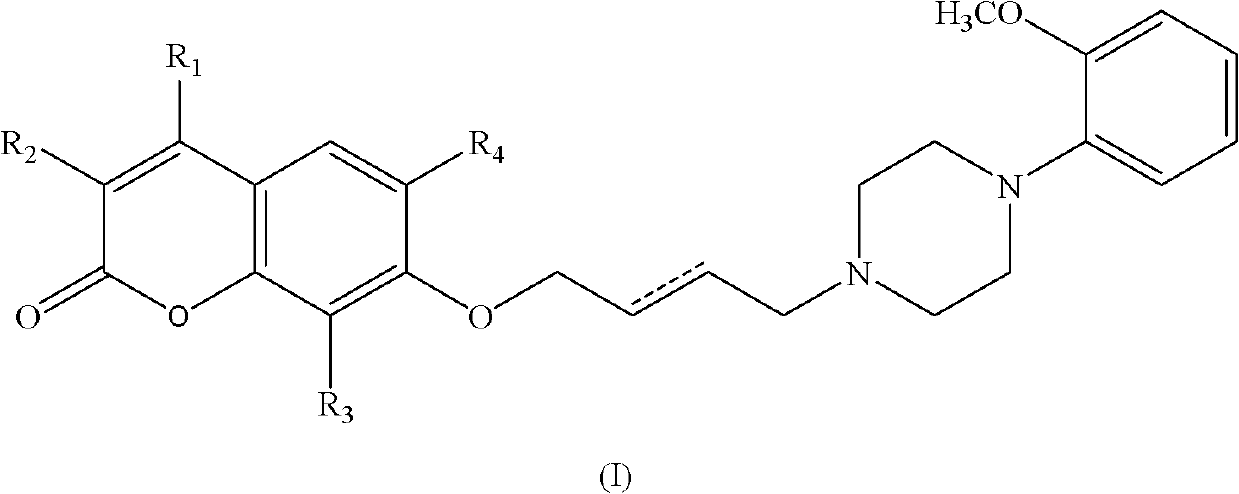
Substituted benzopyrone derivatives and applications thereof
A technology of benzopyrone and benzopyran, which is applied in the field of substituted benzopyrone derivatives, can solve the problems of EPS side effects, QT gap prolongation, and low ratio, and achieve good anti-schizophrenia activity , the effect of improving negative symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1, 7-(4-(4-(2-methoxyphenylpiperazine)-n-butoxy))--2hydro-benzopyran-2-one (1);
[0059]
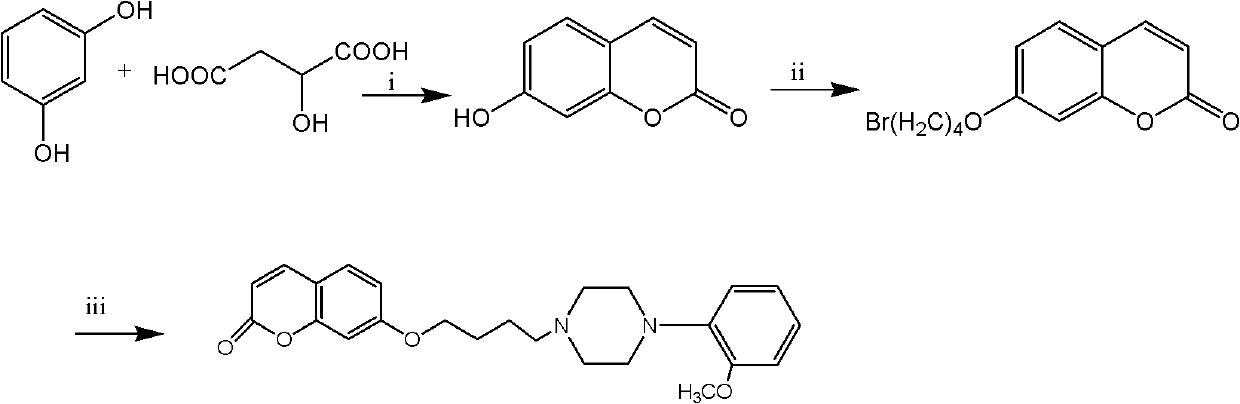
[0060] 1) Take resorcinol 5.5g, d, 1-malic acid 6.7g, add 70% HClO 4 50ml, heated to 90°C to react, the solution gradually became clear, the reaction was completed after 4 hours, cooled to room temperature, the reaction solution was poured into the ice-water mixture, a large amount of solids precipitated, filtered, the filter cake was washed with 95% ethanol Recrystallization gave 4.5 g of white crystals with a melting point of 226-228° C. and a yield of 60.8%.
[0061] 2) Take 5 g of the first step product, 6 g of anhydrous potassium carbonate, 50 ml of acetone, and 8.2 g of 1,4-dibromobutane, heat and reflux for 6 hours, cool to room temperature, filter, and evaporate the solvent to obtain a light yellow oil , and passed through the column to obtain 5.4 g of a white solid with a melting point of 55-57° C. and a yield of 60.7%.
[0062] 3) Take 0.52 g of the second s...
Embodiment 2
[0063] Example 2, 7-(4-(4-(2-methoxyphenylpiperazine)-n-butoxy))-4-methyl-2hydro-chromen-2-one (2)
[0064]
[0065]1) Take 30ml of concentrated sulfuric acid, stir in an ice bath, add 5.5g of resorcinol, dropwise add 9.2g of ethyl acetoacetate, the solution turns from light yellow to yellow, after 18 hours the reaction is complete, pour the reaction solution into the ice-water mixture , a white solid was precipitated, filtered, and the filter cake was washed with water until neutral, and recrystallized with 75% ethanol to obtain 8.5 g of white crystals with a melting point of 186-188°C and a yield of 73.9%.
[0066] 2) Take 5g of the first step product, 6g of anhydrous potassium carbonate, 50ml of acetone and 8.7g of 1,4-dibromobutane, heat to reflux for 4 hours, cool to room temperature, filter, and evaporate the solvent to obtain a light yellow oil , 6.5 g of white solid was obtained through column, the melting point was 58-60° C., and the yield was 77.8%.
[0067] 3) T...
Embodiment 3
[0069] Example 3, 7-(4-(4-(2-methoxyphenylpiperazine)-n-butoxy))-4-phenyl-2hydro-chromen-2-one (3)
[0070] 1) Get resorcinol 5.5g, ethyl benzoyl acetate 9.6g, then add 30ml phosphoric acid, stir at room temperature, the solution turns from light yellow to yellow, after 12 hours the reaction is complete, the reaction solution is poured into the ice-water mixture, A large amount of solids precipitated, filtered, washed the filter cake with water, and recrystallized with 95% ethanol to obtain 9.3 g of white crystals with a melting point of 237-239°C and a yield of 80.9%.
[0071] 2) Take 4.8g of the first step product, 6g of anhydrous potassium carbonate, 50ml of acetone and 8.4g of 1,3-dibromopropane, heat to reflux for 4 hours, cool to room temperature, filter, spin to dry the solvent, and pass through the column to obtain a white solid 5.6g, melting point 67-69°C, yield 78.0%.
[0072] 3) Take 0.5 g of the product of the second step, add 0.6 g of 1-(2-methoxyphenyl) piperazi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com