Magnetic resonance system and method for comprehensive implantable device safety tests and patient safety monitoring
An implanted device and magnetic resonance technology, applied in the field of magnetic resonance technology, can solve the problems of insecurity, increased complexity of the implanted device, and inability to detect local heating and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
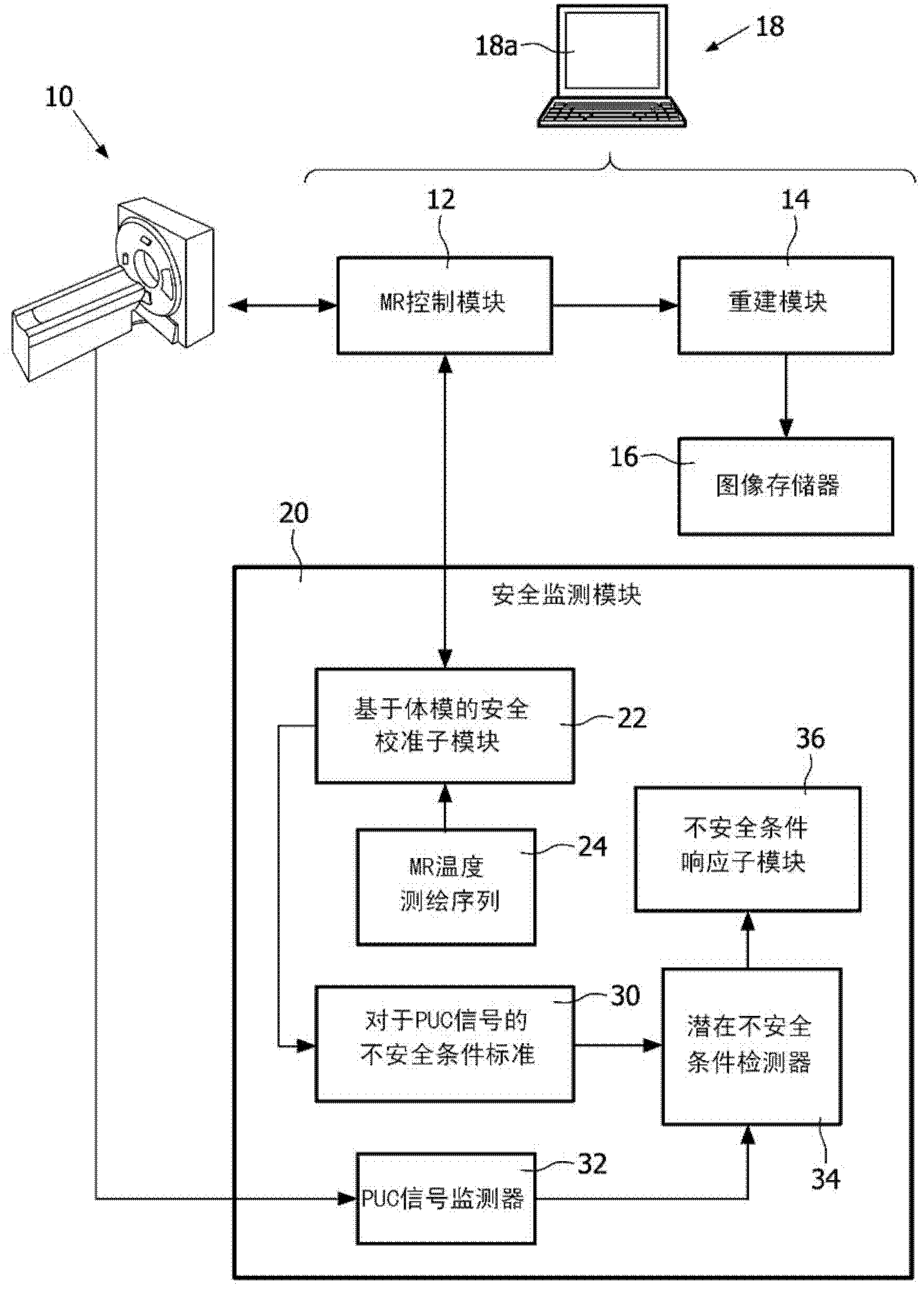
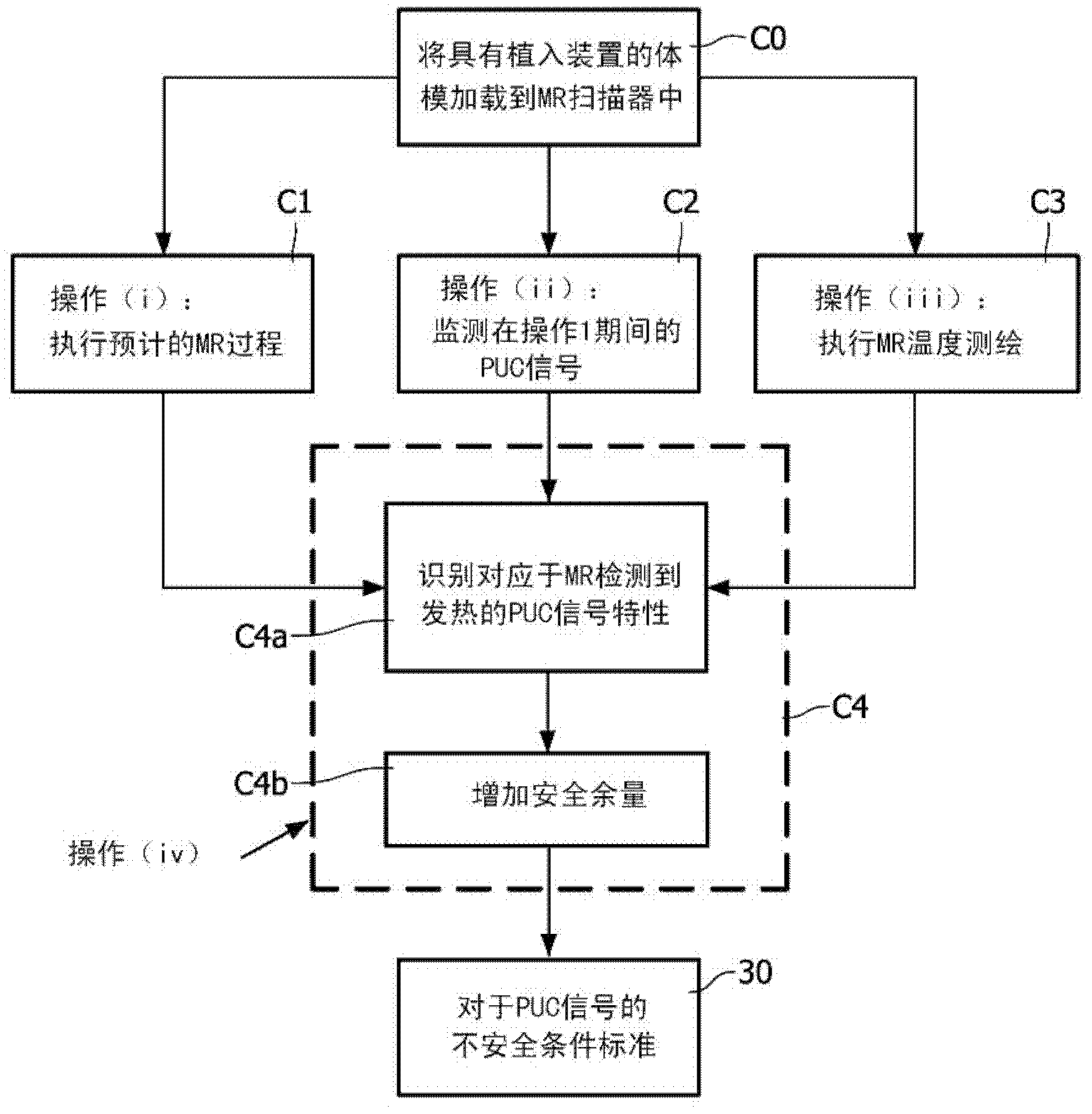
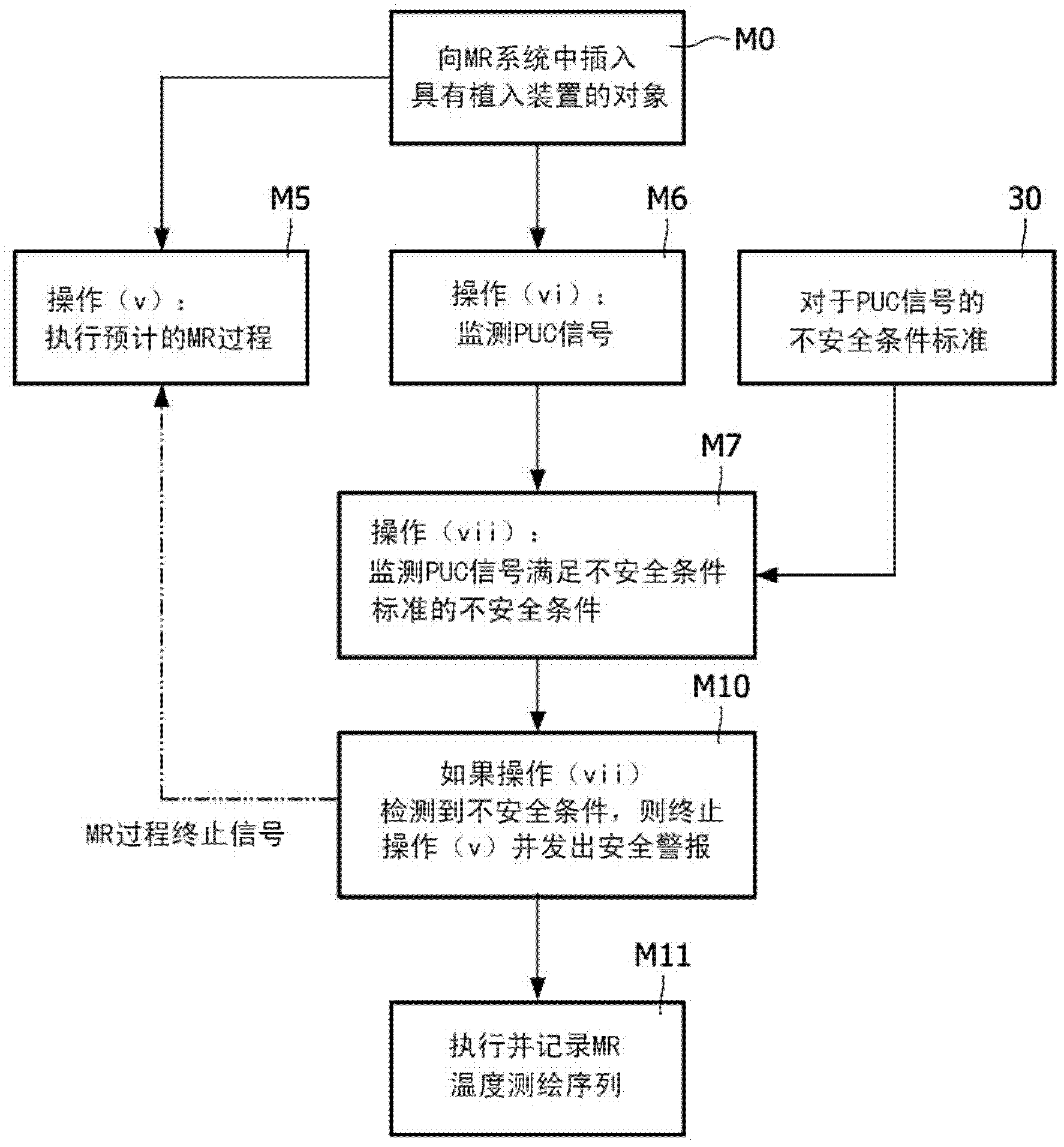
[0020] refer to figure 1 , the magnetic resonance system includes a magnetic resonance scanner 10, such as the illustrated Achieva TM Magnetic resonance scanner (available from Koninklijke Philips Electronics N.V., Eindhoven, The Netherlands) or Intera TM or Panorama TM A magnetic resonance scanner (both also available from Koninklijke Philips Electronics N.V.), or another commercially available magnetic resonance scanner or a non-commercial magnetic resonance scanner, etc. In an exemplary embodiment, the magnetic resonance scanner includes internal components (not shown), such as generating static (B 0 ) magnetic field superconducting or resistive main magnet, for superimposing the selected magnetic field gradient on the static magnetic field group of magnetic field gradient coil windings, for the selected (typically 1 H MRI, but also thought of as 1 H Magnetic resonance as an alternative or supplement to another magnetic resonance nucleus) Frequency generating radio freq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com