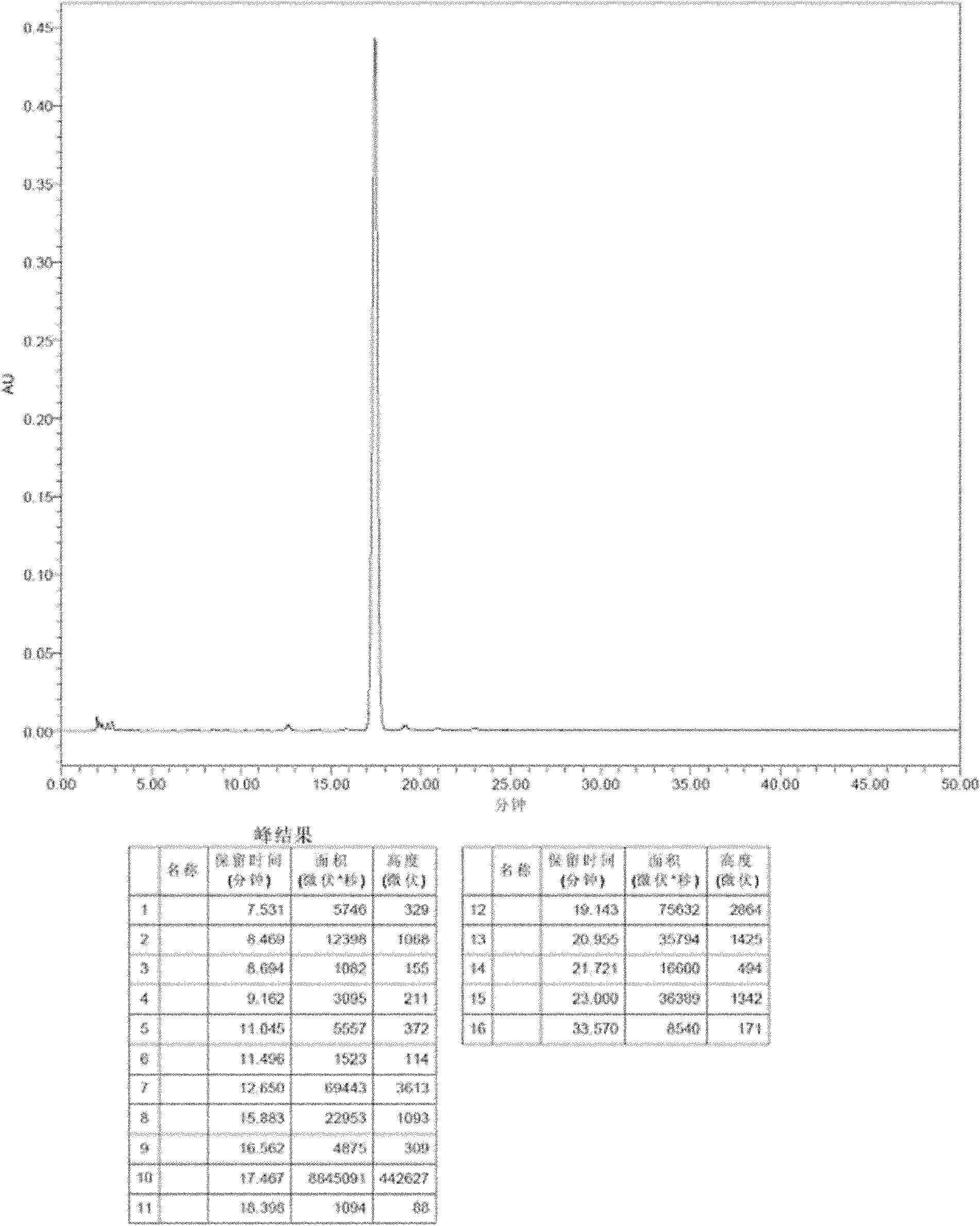Liquid pharmaceutical composition containing echinocandin antifungal agent micafungin
A composition and antifungal technology, applied to the compound represented by the formula: , in the field of liquid pharmaceutical compositions for treating and/or preventing fungal infections, can solve the problems of long production cycle, increased risk of drug use, complicated production process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0047] Micafungin liquid formulation preparation
[0048] Take 30ml of water, add 75μl of glacial acetic acid, and adjust the pH to 5.5 with 1M sodium hydroxide. Dissolve 12.0 g of trehalose in the buffer solution, then add 1.25 g of micafungin sodium, stir gently to dissolve, add water to 50 mL, and filter with a 0.22 μm membrane. The composition of the composition (Formulation 3) is as follows:
[0049] Micafungin Sodium 25mg / ml
[0050] Trehalose 240mg / ml
[0051] Glacial acetic acid 1.5mg / ml
[0052] NaOH Adjust to pH5.5
[0053] The prepared solution was dispensed into 10mL vials at a rate of 2.5mL / bottle, fully stoppered, and capped. The resulting liquid product was subjected to the same stability investigation as that of Comparative Example 1.
Embodiment 4
[0055] Micafungin liquid formulation preparation
[0056]The preparation process is similar to Example 3, except that in the preparation process, the stabilizing agent is selected between trehalose, sucrose, lactose or maltose, and the pH regulator used is selected between acetate, phosphate or citrate , even do not add any additional pH regulator, thus obtaining different formulations, the composition of each composition formulation is as follows:
[0057]
[0058] The composition of each formulation was also subjected to the stability investigation described in Comparative Example 1.
Embodiment 5
[0060] Micafungin liquid formulation preparation
[0061] After the samples of Comparative Example 1, Comparative Example 2, Example 3 and Example 4 were tested for stability, the active substances were analyzed by HPLC.
[0062] The results of the 4-week stability investigation at 70°C are shown in the table below:
[0063]
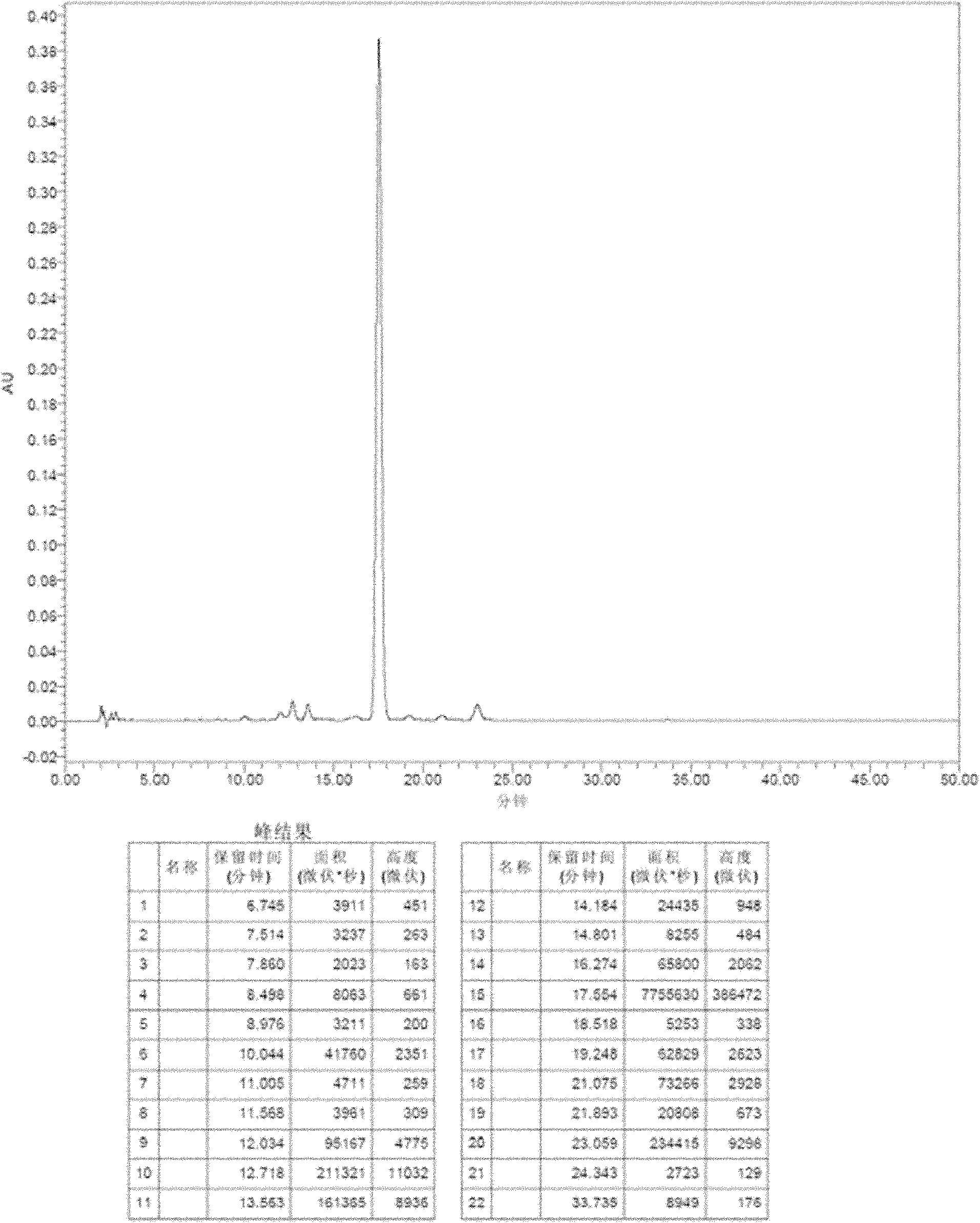
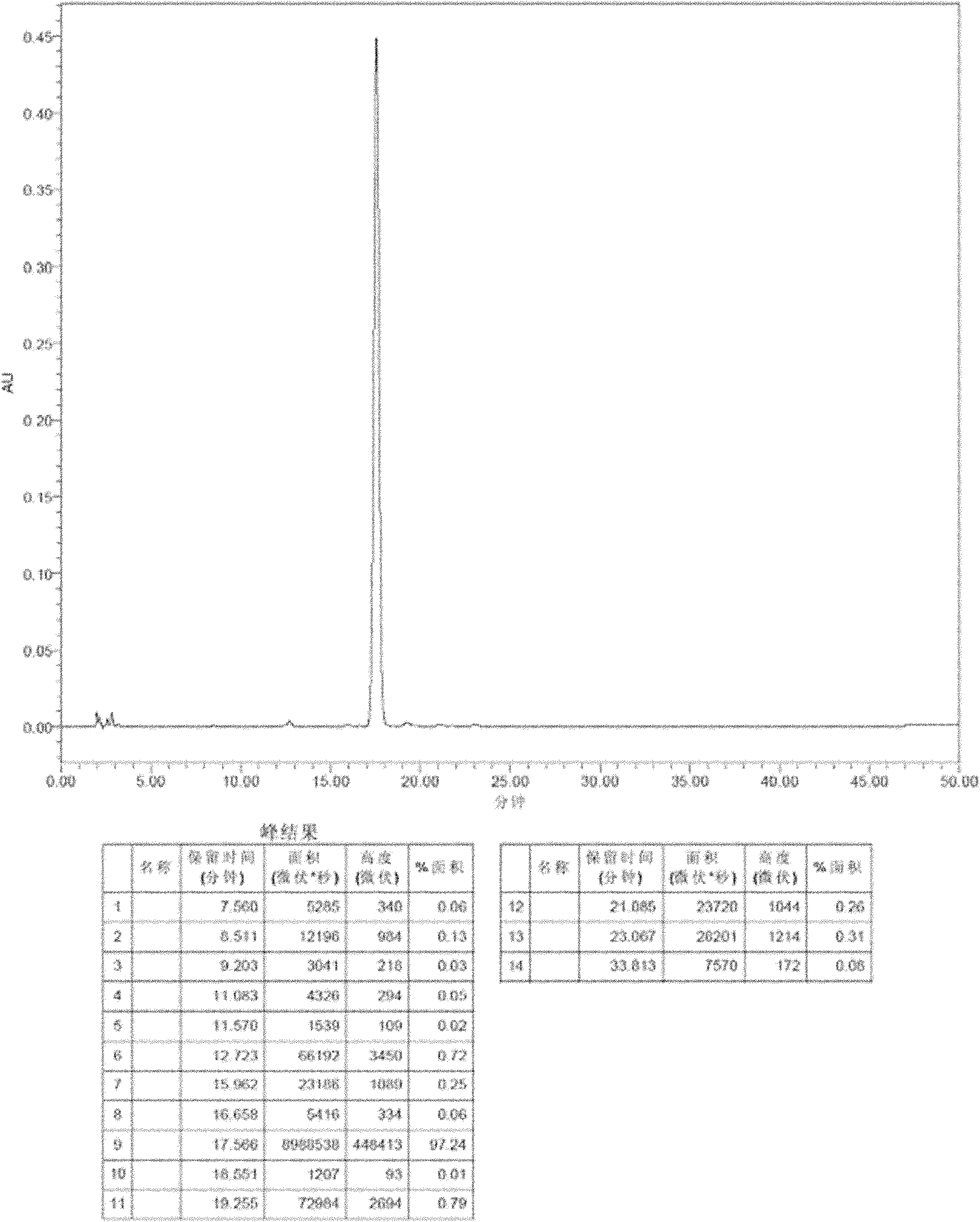
[0064] As can be seen from the above table, the liquid formulation using trehalose, sucrose, lactose or maltose or a combination thereof as a stabilizer, and the weight ratio of the stabilizer to micafungin sodium is 100:1-1:20, preferably 20: In the case of 1-1:5, it has good stability. The HPLC analysis chromatogram of formula 1 and 6 is attached Figure 1-4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com