Preparation for inhibiting immunity and treating graft-versus-host diseases (GVHD) and preparation method of preparation
A graft-versus-host and preparation technology, which can be used in drug combinations, animal cells, pharmaceutical formulations, etc., can solve problems such as enhanced inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Preparation of monocytes.
[0032] Add density gradient agent Histopaque-1077 (Sigma) to the extracted bone marrow or peripheral blood leukocyte suspension purchased directly from the blood bank, and add 30mL bone marrow extract or peripheral blood leukocyte suspension to every 15mL Histopaque. The bone marrow extract or peripheral blood leukocyte suspension was centrifuged at room temperature for 30 minutes at a speed of 400G in the presence of a gradient agent. After the layers are separated, the opaque layer in the middle is aspirated with a sterile disposable needle, which is the suspension rich in myeloid mononuclear cells.
Embodiment 2
[0033] Example 2. Acquisition of MSC cells.
[0034] The method for obtaining type I MSC cells: the bone marrow or peripheral blood mononuclear cells were added to DMEM culture medium with 10% human serum albumin or autologous serum at a rate of 1×10 per square centimeter. 6 The density of cells was cultured for 2 days, the suspended cells were removed, and the adherent cells were cultured for 21 days. The obtained type I MSCs are spindle-shaped and have typical MSC characteristics, that is, they express a large number of cell surface receptors CD90, CD105 and CD73, and do not contain CD14, CD34, CD45 and other hematopoietic stem cell and monocyte receptors.
[0035] The method for obtaining type II MSC cells: the bone marrow or peripheral blood mononuclear cells were added with 10% human serum albumin or autologous serum in DMEM culture medium at a rate of 1×10 per square centimeter. 6 The density of cells was cultured for 2 days, and magnetic bead sorting was carried out by...
Embodiment 3
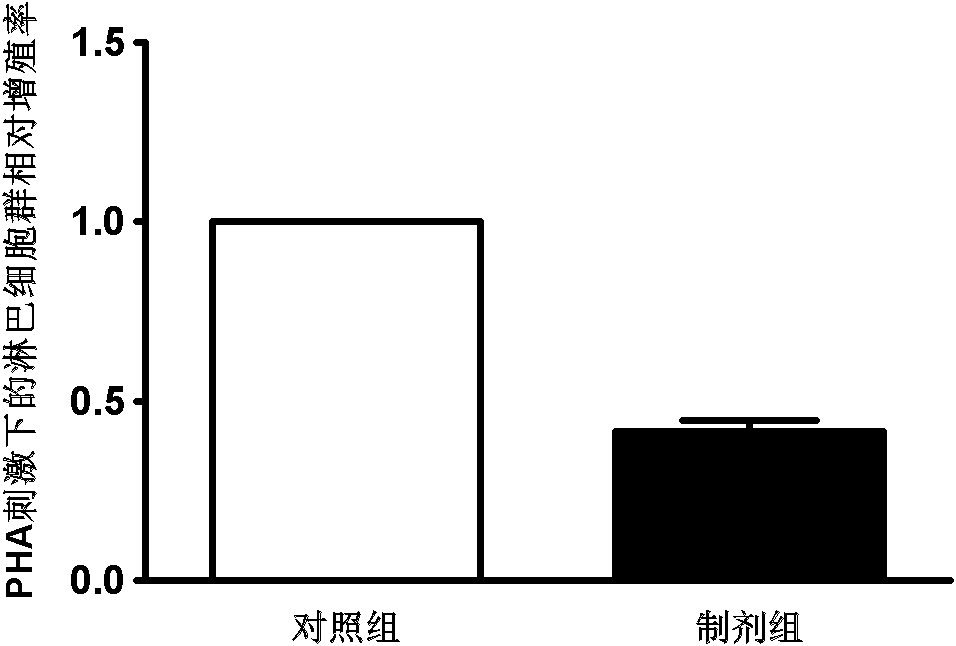
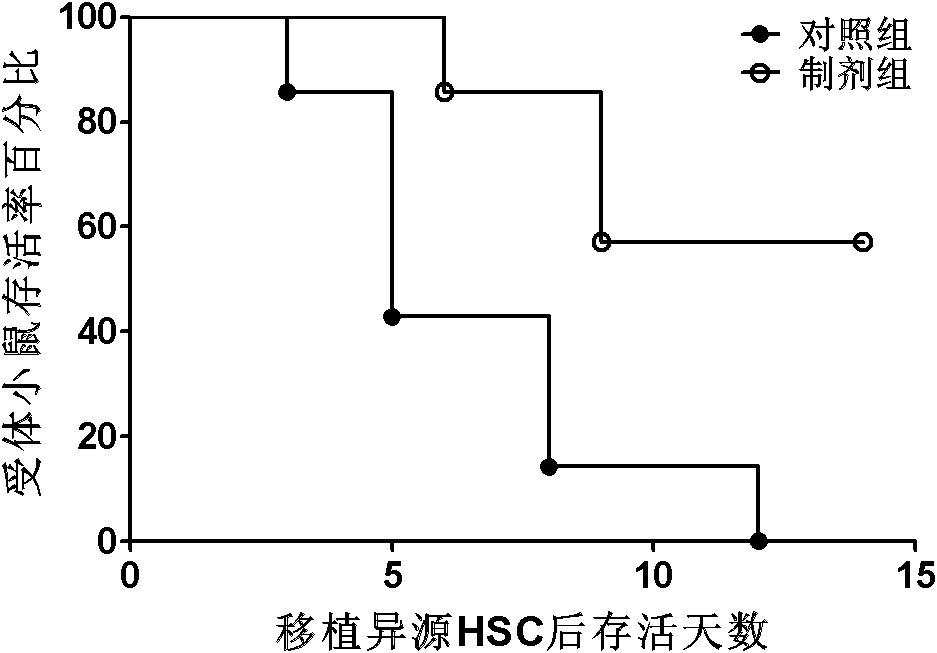
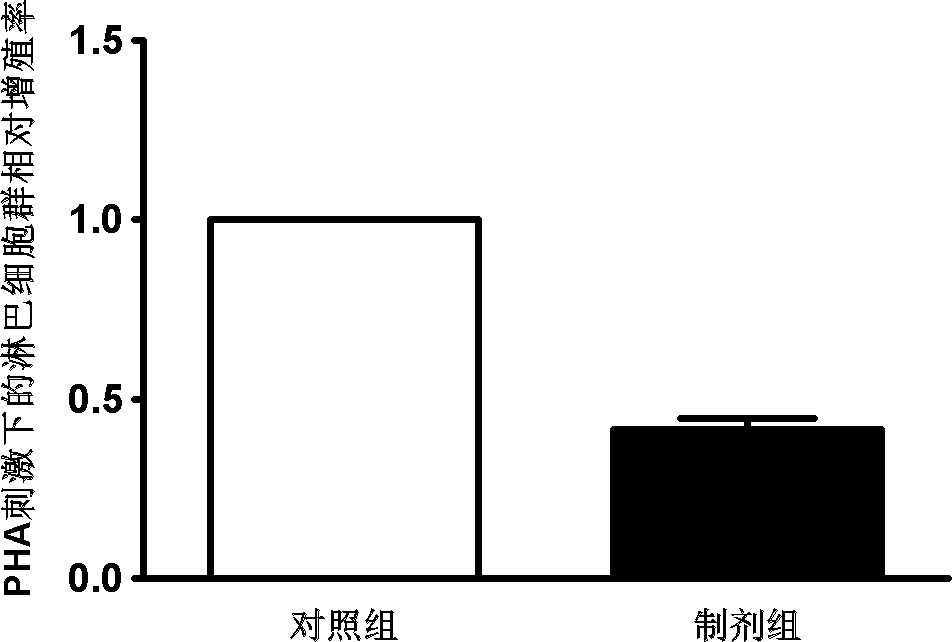
[0037] Example 3. Obtaining preparations for suppressing immunity and treating graft-versus-host disease (GVHD).
[0038] The various types of MSCs obtained in Example 2 were placed in a culture medium without any growth factors, and were cultured in 0.9% medical saline for 2 days in an environment with an oxygen concentration of 0.5% (2 × 10 per square centimeter). 5 cells), and 1% medical human serum albumin or autologous serum can be added. After the culture was completed, the cell-free medium rich in cell growth factors was collected, and the adherent MSC cells were discarded. The collected cell-free medium was passed through a filter with a pore size of 0.2 microns to remove cell impurities and debris, and then stored in a -80°C freezer for later use. As the case may be, every 1x10 6 Each MSC cell can prepare 2-5 ml of the preparation for suppressing immunity and treating graft-versus-host disease (GVHD).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap