Simplified structure of three asprosin, its preparation method, its pharmaceutical composition and use
A compound and halogen technology, applied in the field of medicine, can solve problems such as limited sources, complex structures of natural products, and difficult preparations, and achieve excellent results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
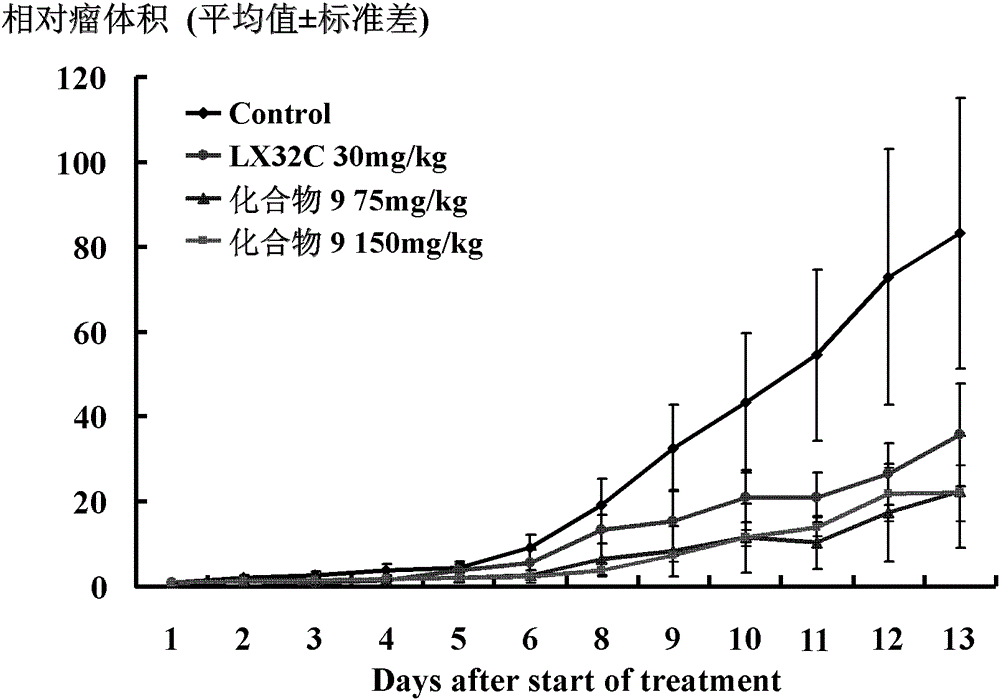
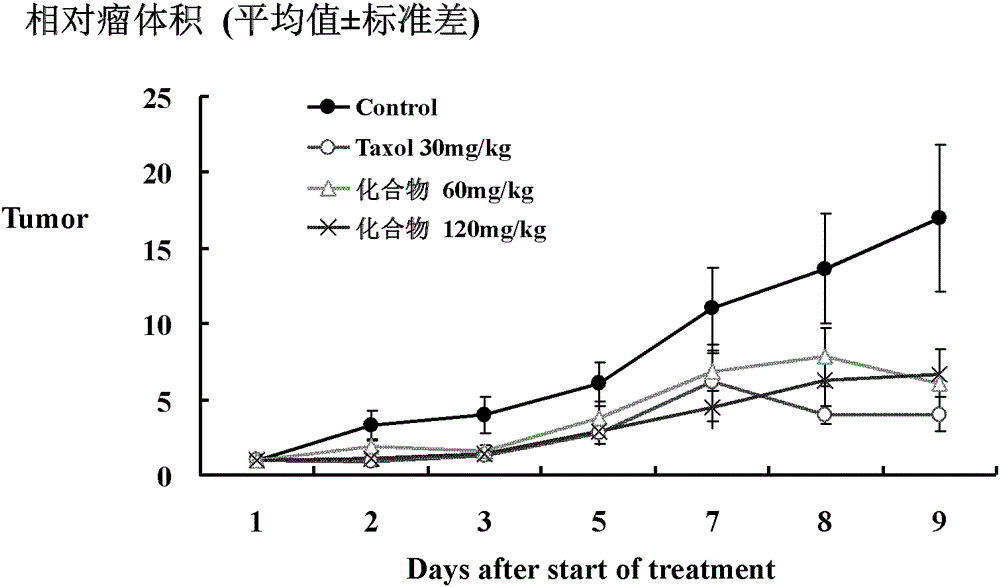
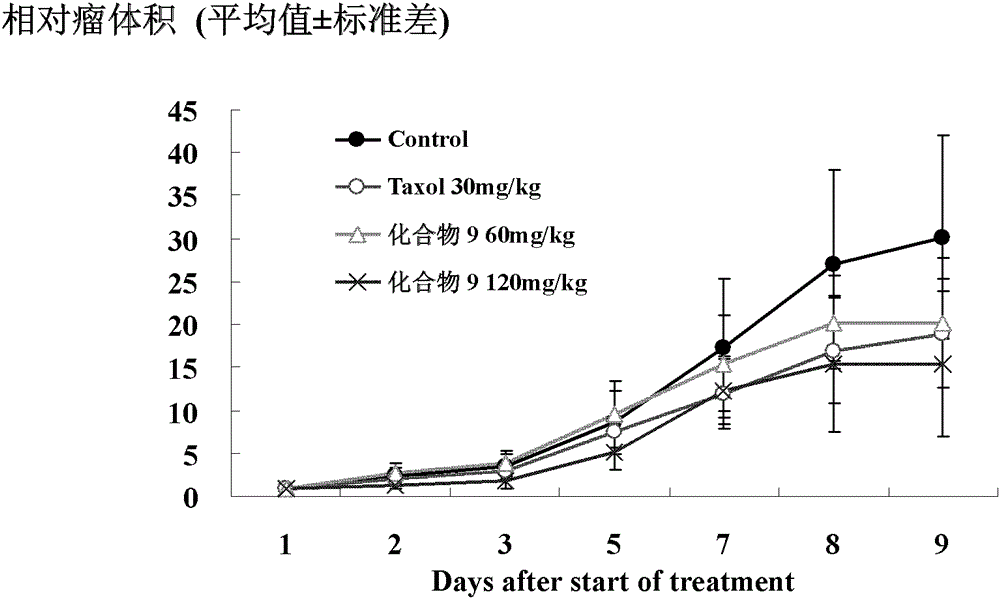
Image
Examples
Embodiment 1
[0081] Step A: Add o-methoxyphenol 10g (80.56mmol), sodium hydroxide 5.76g (144mmol), methanol 100mL in a 250mL round bottom flask, add elemental iodine 20.4g (80.3mmol) at -4°C, and at this temperature After reacting for 1 hour, TLC showed that it was complete. Stop the reaction, pour into water, extract with ethyl acetate, wash with sodium dithionite, wash with saturated sodium chloride, dry with anhydrous sodium sulfate, filter, evaporate the solvent, and obtain 10.6 g of the crude product on a silica gel short column. rate 53%
[0082]
[0083] 1 HNMR (300M, CDCl 3 , δppm) 7.18 (d, 1H, J=8.4Hz, Ar-H), 6.86 (s, 1H, Ar-H), 6.69 (d, 1H, J=
[0084] 8.1Hz, Ar-H), 5.57(s, 1H, OH) 3.88(s, 1H, -OCH 3 )
[0085] Step B: 110.6g (42.4mmol) of compound was added to a 200mL round bottom flask, dissolved in 50mL of anhydrous DMF, 22g (159mmol) of anhydrous potassium carbonate was added, and 1.39mL (42.4mmol) of benzyl bromide was slowly added dropwise under ice bath, rt After 2...
experiment example 1
[0153] Experimental example 1. Inhibitory effect of triasprosin-8 and its derivatives on HIF-1 activity
[0154] (1) Method
[0155] Report gene assay
[0156] The T47D cell model transiently co-transfected with pGL2-TK-3HRE / pRL-CMV plasmid was used for detection. pGL2-TK-3HRE is a firefly luciferase plasmid containing HRE (HIF-1 binding response element), and pRL-CMV is a Renilla luciferase plasmid. Under hypoxic conditions, pGL2-TK-3HRE highly expressed firefly luciferase in cells, but pRL-CMV was not affected.
[0157] 1. Human breast cancer cells T47D (ATCC) use RPMI-1640 containing 10% FBS, penicillin (100IU / ml), streptomycin (100g / ml) in normal oxygen 21% O 2 , 5%CO 2 cultured under conditions. T47D cells were seeded in 96-well plate, 45000 cells / well, 21% O 2 , 5%CO 2After 48 hours of culture, the fusion degree is about 90-95%. Apply lipofectamine2000 for transient co-transfection, add 0.5 μl lipo2000 to each well, pGL2-TK-3HRE, pRL-CMV respectively 0.2 μg, 0.01 ...
experiment example 2、 3
[0172] Experimental Example 2. Inhibitory Effect of Triasprosin-8 and Its Derivatives on Tumor Cell Growth under Normal Oxygen Conditions
[0173] (1) Method
[0174] Determination of Inhibitory Effects of Triasprosin and Its Derivatives on Tumor Cell Growth by MTT Method
[0175] Digest the cells in the logarithmic growth phase with 0.25% trypsin-EDTA, prepare a single-cell suspension with a certain concentration, and inoculate 100-2000 cells / well in a 96-well plate according to the difference in cell growth rate, each well Add 100 μL of cell suspension. After 24 hours, add fresh medium containing different concentrations of compounds and corresponding solvent controls, add 100 μL to each well (DMSO final concentration 50 .
[0176] Inhibition rate (%)=(average OD value of the control group-average OD value of the administration group) / average OD value of the control group×100%
[0177] (2) Results
[0178] Table 4. Inhibitory effect of triasprosin-8 on tumor cell growth ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com