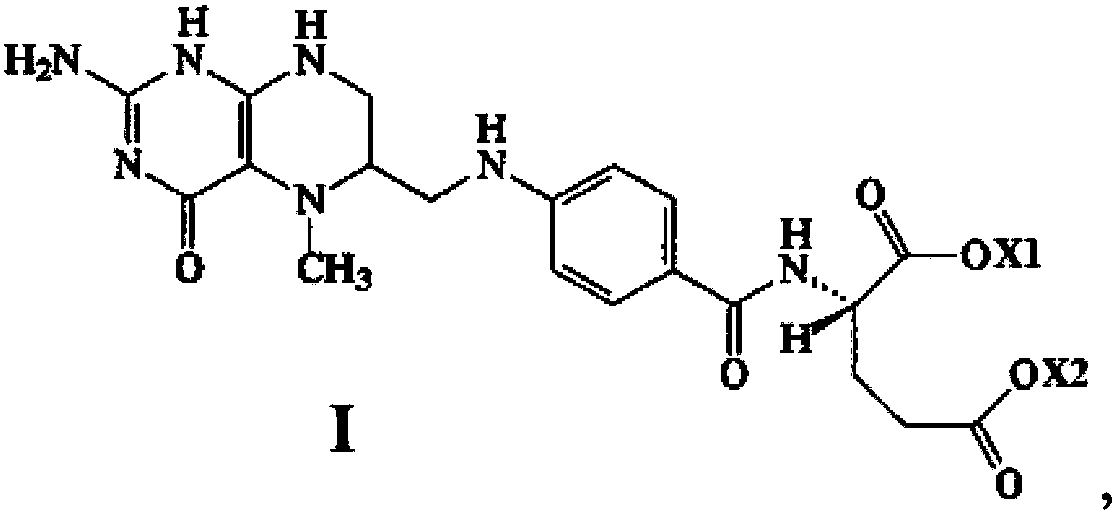
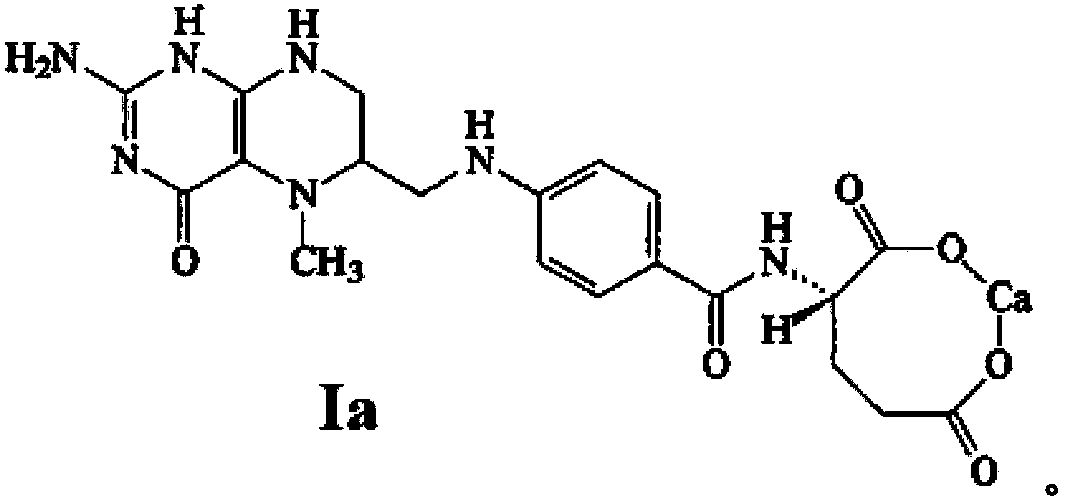
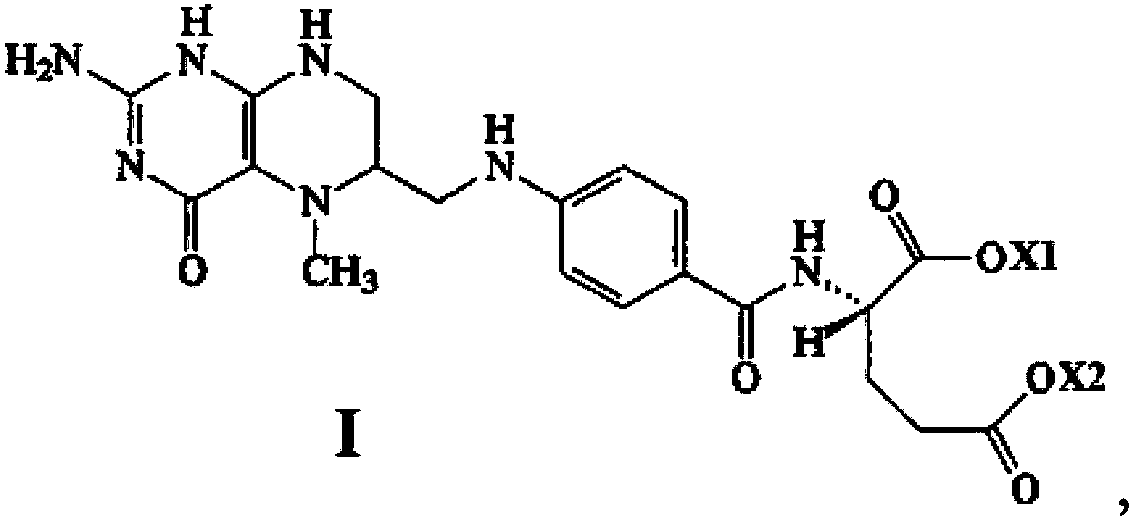
Stable medicine composition of 5-methyltetrahydrofolic acid or salt thereof
A technology of methyltetrahydrofolate and composition, which is applied in the direction of drug combination and metabolic diseases, and can solve problems such as content decline, poor stability, and easy discoloration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: the preparation of 5-methyltetrahydrofolate for injection (1000 bottle amount)
[0092]
[0093] Process: Take about 1.5L of water for injection, add mannitol, arginine and calcium 5-methyltetrahydrofolate, stir to dissolve, adjust the pH value to about 7.5 with 1mol sodium hydroxide solution, add water for injection to 2L, and dissolve the solution Sterile filtration is carried out under sterile conditions, the solution is divided into 2ml, and freeze-dried. Each bottle contains 50mg of 5-methyltetrahydrofolate.
Embodiment 2
[0094] Embodiment 2: the preparation of 5-methyltetrahydrofolate for injection (1000 bottle amount)
[0095]
[0096] Process: Take about 1.5L of water for injection, add mannitol, isoleucine, arginine and calcium 5-methyltetrahydrofolate, stir to dissolve, adjust the pH value to about 7.5 with 1mol sodium hydroxide solution, add water for injection to 2L, the solution was sterilized and filtered under aseptic conditions, the solution was divided into 2ml, and freeze-dried. Each bottle contains 50mg of 5-methyltetrahydrofolate.
Embodiment 3
[0097] Embodiment 3: Preparation of 5-methyltetrahydrofolate aqueous injection preparation (1000 bottles)
[0098]
[0099] Process: Process: Take about 1.5L of water for injection, add sodium chloride, methionine and calcium 5-methyltetrahydrofolate, stir to dissolve, adjust the pH value to about 7.5 with 1mol sodium hydroxide solution, add water for injection to 2L, and The solution is sterilized and filtered under aseptic conditions, and the solution is divided into 2ml. Each bottle contains 50mg of 5-methyltetrahydrofolate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com