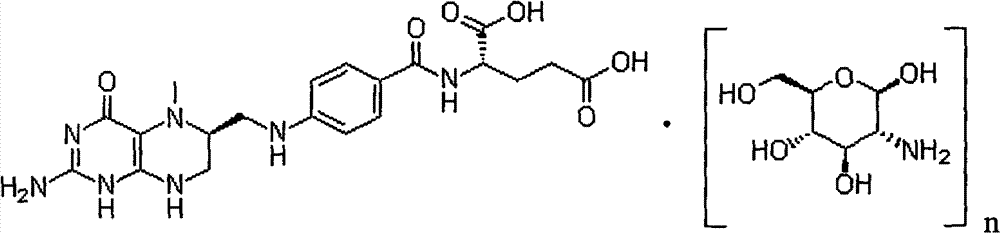
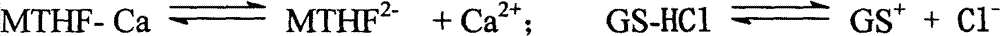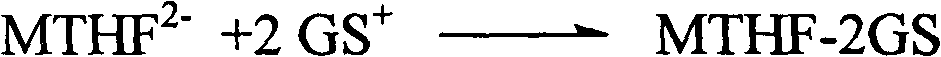Method for reacting L-5-methyltetrahydrofolic acid with organic base to form salt
A technology of calcium methyltetrahydrofolate and organic bases, applied in the directions of organic chemistry, chemical instruments and methods, amino sugars, etc., can solve problems such as poor stability and low yield, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Under nitrogen flow, in a 1000ml three-necked flask, suspend 49.7g (0.1mol) of L-calcium tetrahydrofolate in 200ml of ethanol, and take another 43.1g (0.2mol) of glucosamine hydrochloride dissolved in 200ml of water. While stirring, slowly add to the ethanol suspension of L-calcium tetrahydrofolate, keeping the temperature at 0-20°C; stir the above mixture at room temperature for 8 hours, add dropwise 200ml of acetone or 300ml of absolute ethanol, and then stir for 6 hours; stand for 24 hours; filter, wash with cold ethanol / water, and dry under vacuum at 25-40°C. Recrystallize once if necessary. Obtained 61.8g of crystalline powder of L-5-methyltetrahydrofolate glutamine salt, yield 66.7%, chemical purity 98.6%, [α] 20 D =+54.1° (C=1, water) Optical purity ee≥99.0%.
Embodiment 2
[0028] Under nitrogen flow, in a 1000ml three-necked flask, suspend 49.7g (0.1mol) of L-calcium tetrahydrofolate in 300ml of ethanol, and take 454.5g (0.2mol) of glucosamine sulfuric acid dissolved in 300ml of water, while While stirring, slowly add to the ethanol suspension of L-calcium tetrahydrofolate, keeping the temperature at 20-30°C; stir the above mixture at room temperature for 2 hours, filter, remove calcium sulfate, add dropwise 200ml acetone or 300ml Absolute ethanol, stirring for another 6 hours; standing for 24 hours; filtering, washing with cold ethanol / water, and vacuum drying at 25-40°C. Recrystallize once if necessary. Obtained 62.6g of crystalline powder of L-5-methyltetrahydrofolate glutamine salt, yield 67.5%, chemical purity 98.5%, [α] 20 D =+53.9° (C=1, water) optical purity ee≥99.0%,.
Embodiment 3
[0030] Under nitrogen flow, in a 1000ml three-necked flask, suspend 49.7g (0.1mol) L-calcium tetrahydrofolate in 200ml ethanol, and take another 39.1g (0.2mol) N-methylglucose dissolved in 200ml water Sugar amine, while stirring, is slowly added to the ethanol suspension of L-methyltetrahydrofolate calcium, and the temperature is kept at 0-20°C; the above mixture is fully stirred at room temperature, and after 8 hours, 200ml of acetone or 300ml of anhydrous For ethanol, adjust the pH to 6.5 with dilute hydrochloric acid, and then stir for 6 hours; let stand for 24 hours; filter, wash with cold ethanol / water, and dry under vacuum at 25-40°C. Recrystallize once if necessary. Obtained 61.1 g of L-5-methyltetrahydrofolate meglumine salt crystalline powder, yield 68.9%, chemical purity 98.2%, [α] 20 D =+54.1° (C=1, water) Optical purity ee≥99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com