Pharmaceutical composition containing aspirin, clopidogrel and folic acid compound
A technology of aspirin and clopidogrel is applied in the direction of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., to achieve the effect of reducing risks and reducing drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1. prepares compound aspirin / clopidogrel / folic acid sheet (1000 pieces amount)
[0028] Recipe:
[0029]
[0030]
[0031] Preparation method: pass the auxiliary materials through a 80-mesh sieve, and set aside. Take 100g of aspirin, 75g of clopidogrel, 0.8g of folic acid, 112g of microcrystalline cellulose, and 40g of lactose, mix them evenly, add an appropriate amount of 10% hypromellose, make soft materials, pass through a 20-mesh nylon sieve to make wet granules, 60 Dry at ±5°C for 2 hours, control the water content of the granules to be below 1.5%, sieve the granules with a 20-mesh sieve, mix them evenly with sodium stearyl fumarate, and press into tablets. Pay attention to avoiding light during the preparation process, and the prepared tablets need to be packed in aluminum-plastic blisters and stored away from light. Each of the prepared compound tablets contains 100 mg of aspirin, 75 mg of clopidogrel and 0.8 mg of folic acid.
Embodiment 2
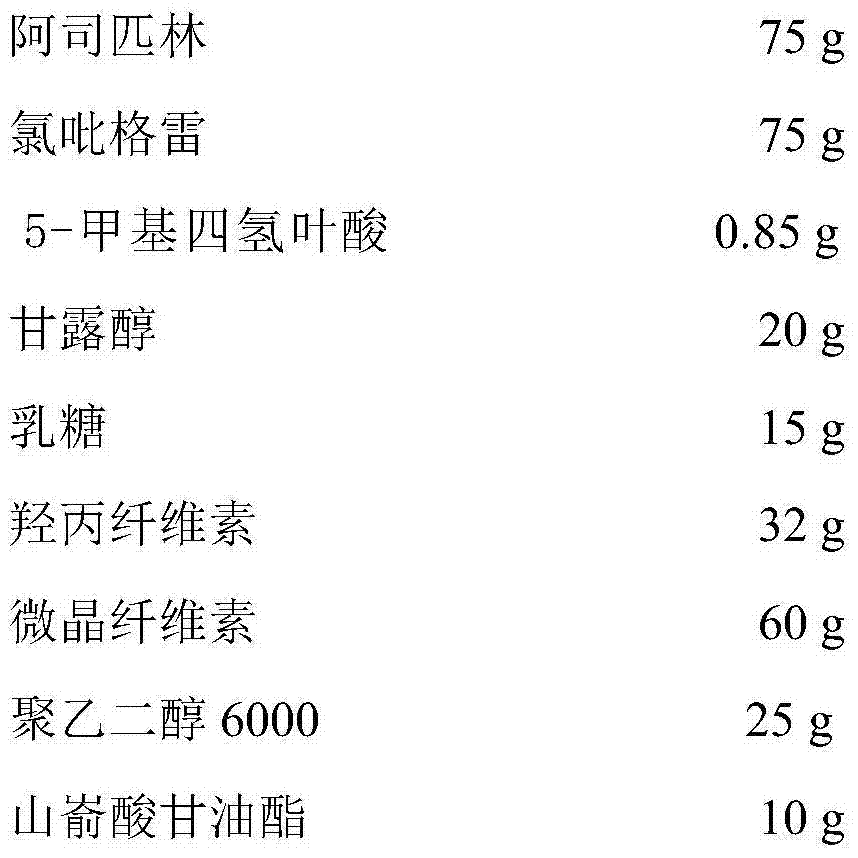
[0032] Embodiment 2. Preparation of compound aspirin / clopidogrel / 5-methyltetrahydrofolic acid film-coated tablets (1000 pieces)
[0033] Recipe:
[0034]
[0035] Coating prescription
[0036] Coating material: film coating premix 9g
[0037] Coating solvent: purified water 66g
[0038] Preparation method: Weigh aspirin, clopidogrel, 5-methyltetrahydrofolate and PEG6000 according to the prescription amount, and set aside. Add the weighed prescription amount of aspirin, clopidogrel, 5-methyltetrahydrofolate and PEG6000 into the granulator, and granulate at 50°C±5°C. Pass through a 30-mesh sieve for granulation to obtain drug granules, weigh hydroxypropyl cellulose, mannitol, lactose, microcrystalline cellulose and glyceryl behenate, put the weighed drug granules and auxiliary materials into a mixer and mix for 20 minutes, The material is discharged to obtain intermediate particles. The intermediate is inspected, and after passing the test, it is pressed into tablets, an...
Embodiment 3
[0039] Example 3. Preparation of Compound Aspirin / Clopidogrel / Folic Acid Capsules (1000 Capsules)
[0040] Recipe:
[0041]
[0042] Preparation method: Take the prescribed amount of folic acid, aspirin, and clopidogrel and mix them uniformly according to the method of equal increase, and set aside; then mix them evenly with mannitol, crospovidone, and calcium hydrogen phosphate, pass through a 80-mesh sieve, and add polyethylene glycol Alcohol 400, appropriate amount of 5% hypromellose solution, make soft material, granulate with 24 mesh sieve, dry at 50°C for about 6 hours, pass through 24 mesh sieve for granulation, control the water content of the granules to 2-3%, and dry the dried The granules are uniformly mixed with the hydrogenated castor oil and talcum powder in the prescribed amount, and the obtained granule intermediate is tested for content, and after passing the test, it is packed into a hollow capsule to obtain the product. Each prepared capsule contains 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com