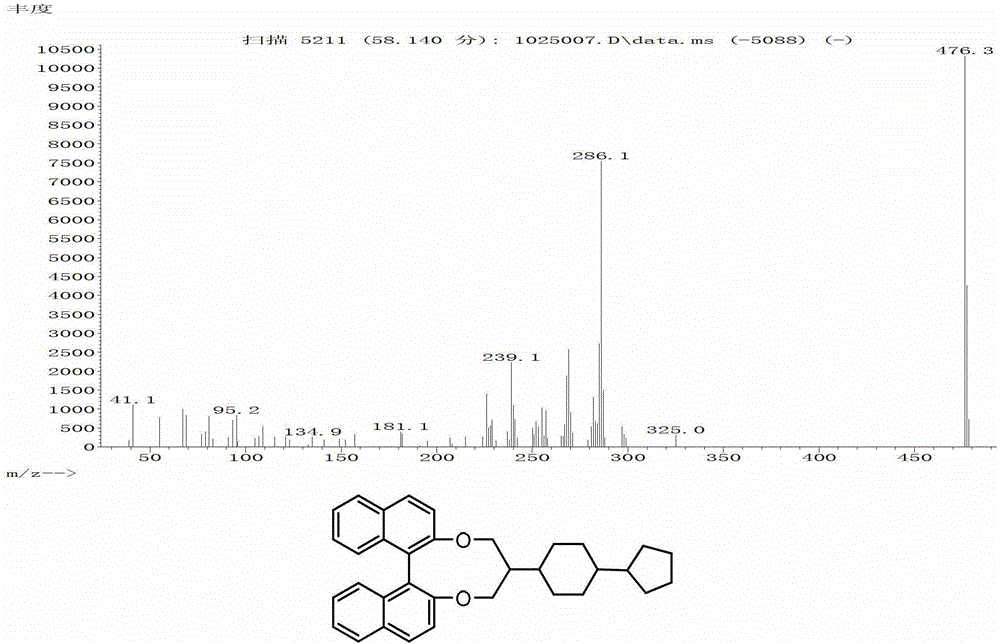
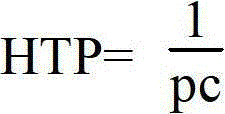Binaphthalene diol chiral compound and its preparation method and application
A technology of compounds and groups, applied in chemical instruments and methods, organic chemistry, nonlinear optics, etc., can solve problems such as low HTP value of chiral additives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086]
[0087] step 1
[0088]
[0089] Add 149g (1mol) cyclopentane bromide and 314.4g (1.2mol) triphenylphosphine to a 1L three-neck flask, heat to 120°C under nitrogen protection, react for 10 hours under stirring, cool down to 100°C, add 400ml of toluene under stirring , the reaction solution was made into a slurry, cooled to room temperature, filtered, and rinsed with toluene to obtain 267g of white crystal bromocyclopentane triphenylphosphine salt with a yield of 65%. DSC:
[0090] step 2
[0091]
[0092] Add 267g (0.65mol) of white crystal bromocyclopentane triphenylphosphine salt into a 2L three-necked flask, add 800ml of tetrahydrofuran, cool down to -5°C, replace the air with nitrogen, and add 73g (0.65mol) of tert-butyl in batches Potassium alkoxide turns yellow. Half an hour later, add 85.8g (0.55mol) monoethylene glycol acetal 1,4-cyclohexanedione solution in 150ml tetrahydrofuran dropwise, and then react at room temperature for 4 hours.
[0093] Pou...
Embodiment 2
[0137]
[0138] As in Steps 6 and 7 of Example 1, the raw material was replaced with 4-propylcyclohexyl alcohol to prepare diethyl 2-(4-propylcyclohexyl)malonate.
[0139] step 1
[0140]
[0141] 85.2 g (0.3 mol) of cis+trans-diethyl 2-(4-propylcyclohexyl)malonate and 63.6 g (0.6 mol) of sodium carbonate were stirred together in 300 ml of ethanol and 100 ml of water overnight.
[0142] Add 300ml of water, dilute hydrochloric acid to adjust the pH to strong acidity, extract with 100ml×2 toluene, wash twice with water, evaporate the solvent under reduced pressure, and recrystallize from toluene to obtain trans-2-(4-propylcyclohexyl)malonic acid as a white solid 21.3g, yield 31%.
[0143] step 2
[0144]
[0145] 5.1g (0.018mol) binaphthyldiol is dissolved in 50ml methylene chloride, then add DCC9.1g (0.044mol), DMAP0.3g, add 4.56g (0.02mol) trans-2-(4 -Propylcyclohexyl) malonic acid, after adding one hour, remove the low-temperature tank, and stir and react at room te...
Embodiment 3
[0154]
[0155] Add 0.127g (0.0055mol) of sodium metal to 100ml of absolute ethanol. After the sodium block disappears, add 2.39g (0.005mol) of the product (2-a) of Example 2. After half an hour, add 0.85g (0.006mol) Iodomethane, reacted at room temperature for 8 hours.
[0156] Add 300ml of water and 50ml of toluene, separate the organic layer, extract the aqueous phase with 50ml of toluene once more, wash the organic phase with water, evaporate the solvent under reduced pressure, and recrystallize with a mixed solvent of ethanol and toluene to obtain 1.88g of white crystals, with a yield of 76%.
[0157] DSC: 150°C
[0158] HPLC: 99.67%
[0159] HTP: 99
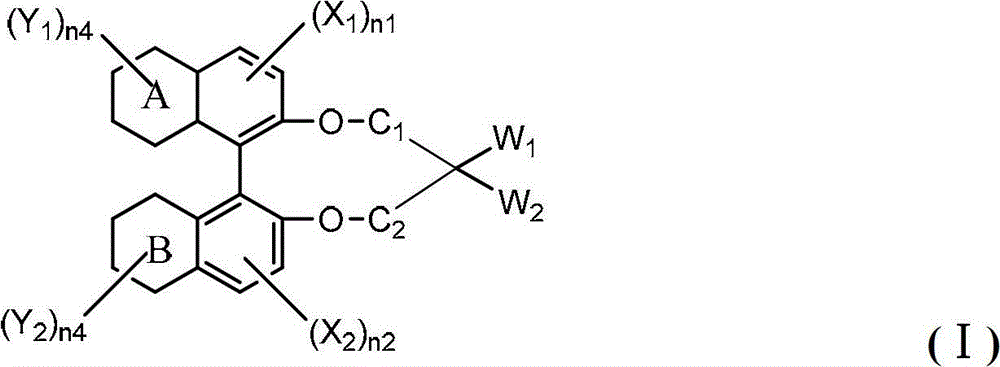
[0160] The raw materials of different substituents are used, prepared according to the above method, and the compound shown in the following formula I is obtained. The HTP value and response time comparison test results of the following compounds are as in Example 1. The resulting product has no substantial difference,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com