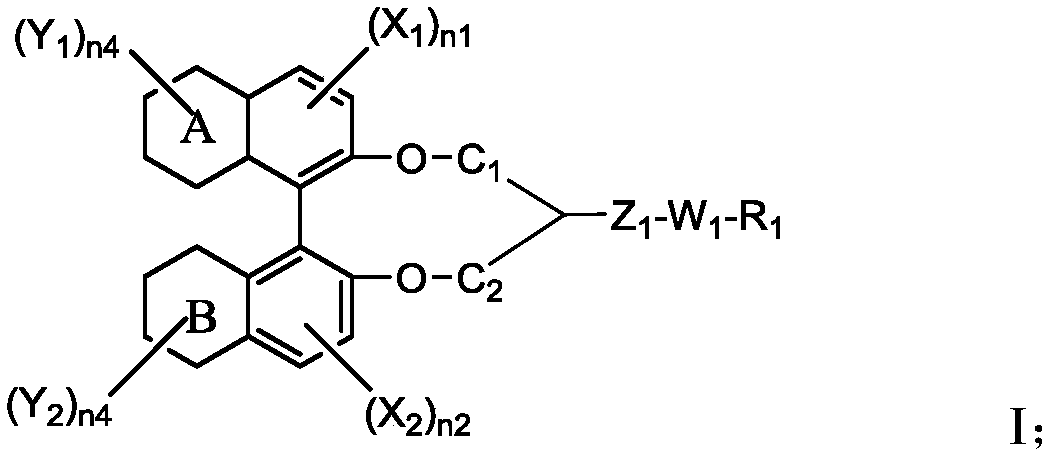
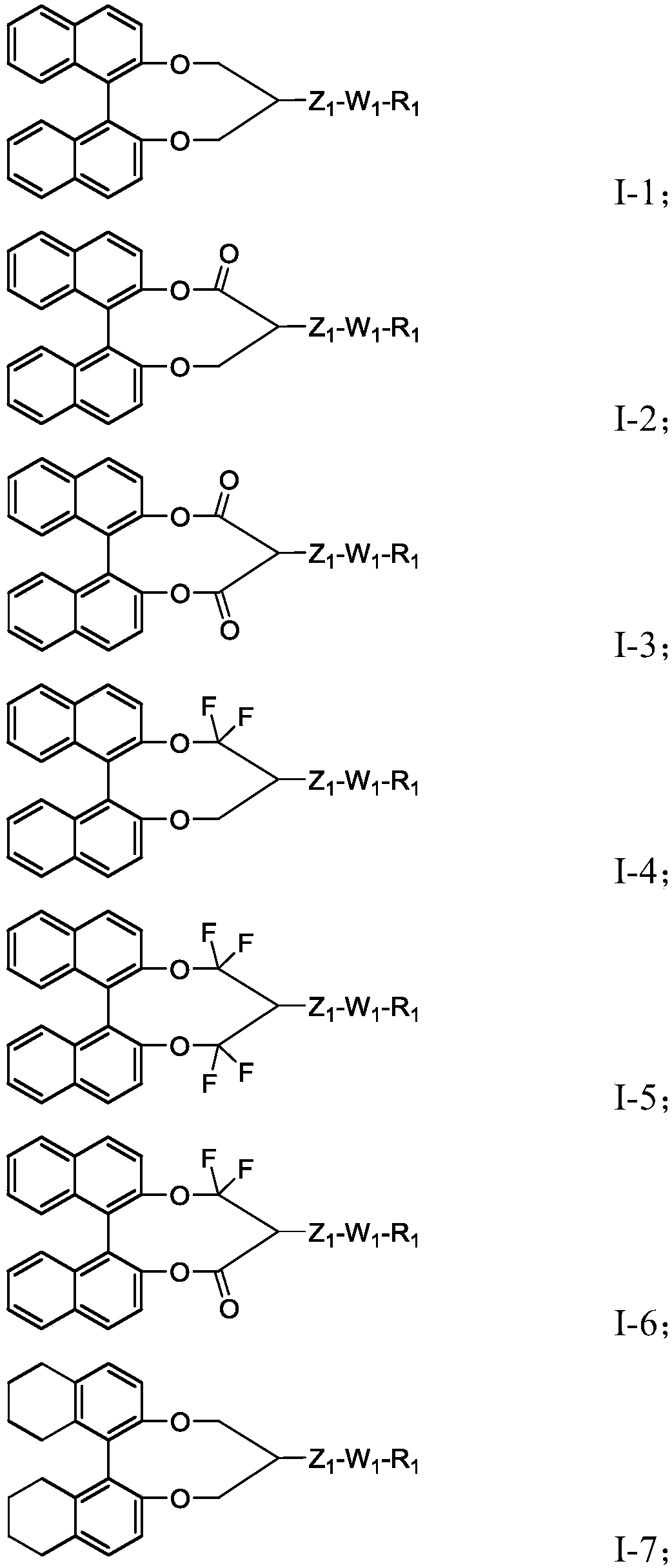
Chiral compound, liquid crystal composition containing the chiral compound, optically anisotropic body, and liquid crystal display device
A technology of optical anisotropy and chiral compounds, applied in nonlinear optics, chemical instruments and methods, optics, etc., can solve the problems of low HTP value of chiral additives, achieve good solubility, reduce synthesis costs, and reduce negative effect of influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
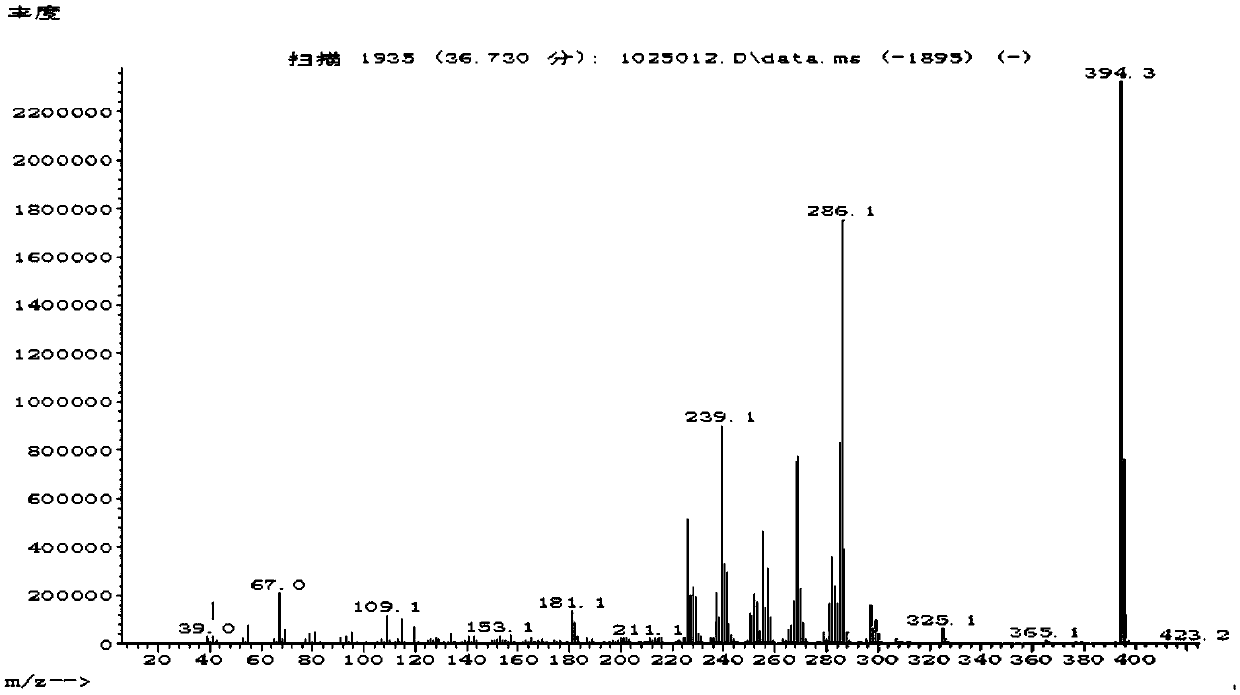
[0062] The structural formula of the chiral compound is shown in the following formula:
[0063]
[0064] Its preparation steps are as follows:
[0065] Step 1, the preparation route is as follows:
[0066]
[0067] The specific operation process of preparation:
[0068] Dissolve 30.7g (0.20mol) of diethyl malonate in 200ml of absolute ethanol, add 4.7g (0.20mol) of sodium metal under nitrogen protection, and after the sodium block disappears, add dropwise 30g (0.187mol) of a colorless liquid bromo Cyclopentane, reflux reaction for 4 hours after addition.
[0069] The reaction solution was lowered to room temperature, poured into 1L of water, extracted with 100ml×3 petroleum ether, combined the petroleum ether phase, washed twice with water, evaporated the solvent under reduced pressure, and distilled under reduced pressure to remove the fraction with a boiling point lower than 95°C / 700Pa. The high boiler was the product, and 31.8 g of diethyl cyclopentylmalonate was ...
Embodiment 2
[0097] The structural formula of the chiral compound is shown in the following formula:
[0098]
[0099] Step 1: Same as Example 1 step 1, the difference is that bromocyclopentane is replaced by 3-propyl bromocyclopentane to prepare 2-(3-propylcyclopentyl) diethyl malonate ester.
[0100] Step 2, the preparation route is as follows:
[0101]
[0102] The specific operation process of preparation:
[0103] 85.2 g (0.3 mol) of 2-(3-propylcyclopentyl) diethyl malonate prepared in step 1 and 63.6 g (0.6 mol) of sodium carbonate were stirred overnight in 300 ml of ethanol and 100 ml of water.
[0104] Add 300ml of water, dilute hydrochloric acid to adjust the pH to strong acidity, extract with 100ml×2 toluene, wash twice with water, evaporate the solvent under reduced pressure, and recrystallize from toluene to obtain 2-(4-propylcyclopentyl)malonic acid as a white solid 21.3 g, yield 31%.
[0105] Step 3, the preparation route is as follows:
[0106]
[0107] The spe...
Embodiment 3
[0114] The structural formula of the chiral compound is shown in the following formula:
[0115]
[0116] Step 1: Same as step 1 of Example 1, the difference is that bromocyclopentane is replaced by bromocyclobutane to prepare diethyl cyclobutylmalonate:
[0117]
[0118] The specific operation process of preparation:
[0119] Dissolve 30g (0.18mol) of diethyl malonate in 200ml of absolute ethanol, add 5.1g (0.22mol) of sodium metal under nitrogen protection, and after the sodium block disappears, add 30g (0.157mol) of colorless liquid bromocyclic Butane, reflux reaction for 4 hours after adding.
[0120] Pour into 1L of water, extract with 100ml×3 petroleum ether, combine the petroleum ether phase, wash twice with water, distill the solvent under reduced pressure, distill under reduced pressure, distill off the fraction with a boiling point lower than 112°C / 920Pa, the high boiling point is the product, 27.8 g of diethyl cyclobutylmalonate was obtained with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com