Method for producing R-alpha-hydroxybutyrate by using 1, 2-butanediol as substrate
A technology of hydroxybutyric acid and butanediol, which is applied in the field of producing R-alpha-hydroxybutyric acid, can solve the problems that the method for producing R-alpha-hydroxybutyric acid has not been reported, and achieves easy separation and purification, simple reaction system, Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
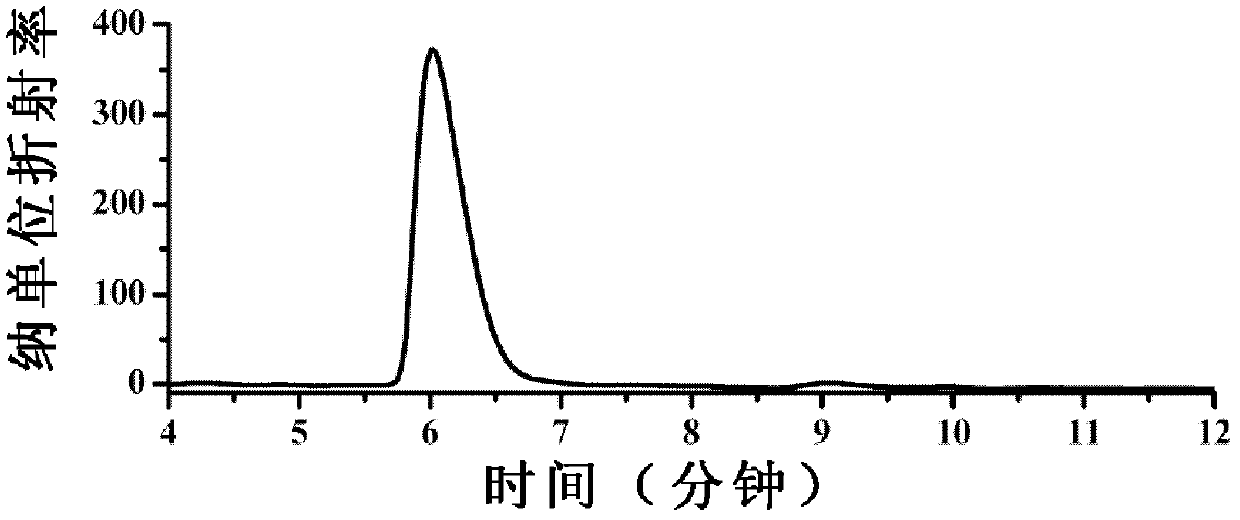
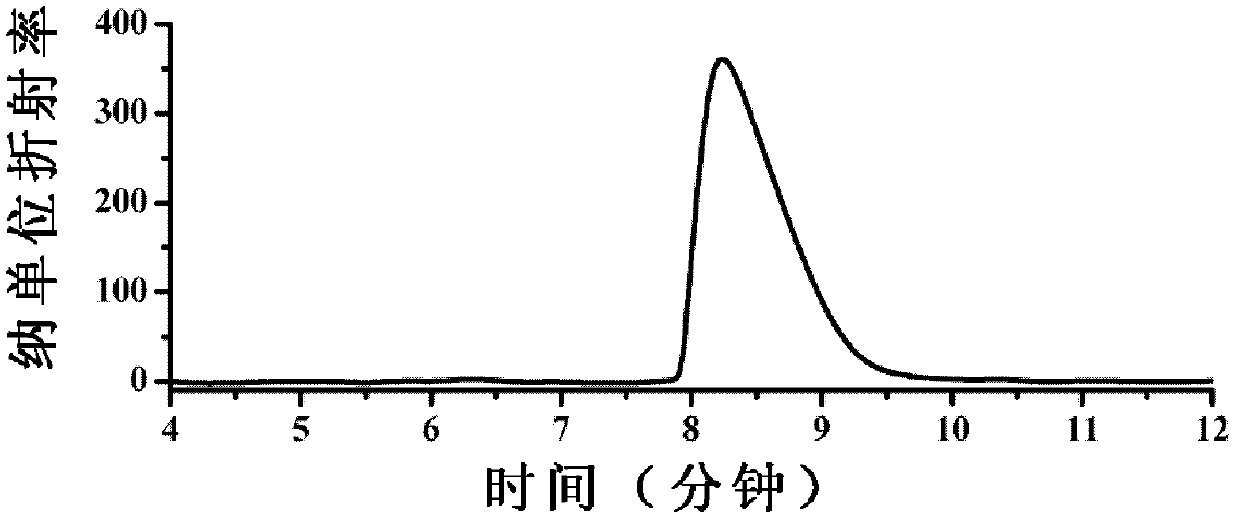
Image
Examples
Embodiment 1
[0042] (1) Preparation of biocatalyst
[0043] Gluconobacter oxidans DSM 2003 (purchased from the German Microorganism Collection Center) was selected, cultivated by conventional methods, separated and collected, washed with pH 7.4 potassium phosphate buffer for 3 times, resuspended in deionized water, and the concentration of the bacteria was increased. When it reaches 200 grams of wet cells / liter, the obtained complete cell suspension is the biocatalyst, which is stored at 4°C for later use;
[0044] Among them, the above-mentioned Gluconobacter oxidans is cultivated using the following medium formula: D-sorbitol 80 g / L; yeast powder 24 g / L; ammonium sulfate 5 g / L; potassium dihydrogen phosphate 2 g / L; magnesium sulfate 5 g / L liter; calcium carbonate 10 g / l.
[0045] (2) conversion
[0046] The biocatalyst prepared in the step (1), that is, the complete cell, is mixed with the substrate 1,2-butanediol aqueous solution, so that the concentration of the biocatalyst in the mi...
Embodiment 2
[0052] (1) Preparation of biocatalyst
[0053] Select Gluconobacter oxydans DSM 2003, cultivate it by conventional methods, separate and collect the bacteria, wash the bacteria three times with pH 7.4 potassium phosphate buffer, resuspend the bacteria in deionized water, and make the bacteria concentration reach 200 g wet cells / liter, The obtained complete cell suspension is the biocatalyst and stored at 4°C for later use;
[0054] Among them, the above-mentioned Gluconobacter oxidans is cultivated using the following medium formula: D-sorbitol 80 g / L; yeast powder 24 g / L; ammonium sulfate 5 g / L; potassium dihydrogen phosphate 2 g / L; magnesium sulfate 5 g / L liter; calcium carbonate 10 g / l.
[0055] (2) conversion
[0056] The biocatalyst prepared in the step (1), that is, the complete cell, is mixed with the substrate 1,2-butanediol aqueous solution, so that the concentration of the biocatalyst in the mixture is 100 grams of wet cells / liter, 1,2-butanediol The concentration...
Embodiment 3
[0062] (1) Preparation of biocatalyst
[0063] Select Gluconobacter oxydans DSM 2003, cultivate it by conventional methods, separate and collect the bacteria, wash the bacteria three times with pH 7.4 potassium phosphate buffer, resuspend the bacteria in deionized water, and make the bacteria concentration reach 200 g wet cells / liter, The obtained complete cell suspension is the biocatalyst and stored at 4°C for later use;
[0064] Among them, the above-mentioned Gluconobacter oxidans is cultivated using the following medium formula: D-sorbitol 80 g / L; yeast powder 24 g / L; ammonium sulfate 5 g / L; potassium dihydrogen phosphate 2 g / L; magnesium sulfate 5 g / L liter; calcium carbonate 10 g / l.
[0065] (2) conversion
[0066] The biocatalyst prepared in the step (1), that is, the complete cell, is mixed with the substrate 1,2-butanediol aqueous solution, so that the concentration of the biocatalyst in the mixture is 120 grams of wet cells / liter, 1,2-butanediol The concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com