Application of a kind of quercetin derivative in preparation of antitumor drug
An anti-tumor drug and tumor technology, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of quercetin activity research and clinical application limitations, large intermolecular attraction, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
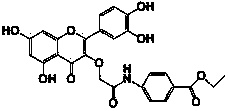
[0013] Example 1 The structural representation of the compound QB
[0014] M.P: 294.9 ~ 296.8 ℃;
[0015] 1 H NMR (400 MHz, DMSO) Δ 12.43 (s, 1H, 5-OH), 10.90 (S, 1H, 7-OH), 10.59 (s, 1H, NH), 9.80 (s, 1H, 4′-OH), 9.45 (s, 1H, 3′-OH), 7.95 (d, j = 8.8 Hz, 2H, 2′-H, 6′-H), 7.83 (d, j = 8.8 Hz, 2H, 2 × pH-H), 7.61-7.54 (m, 2H, 2 × PH-H), 6.91 (d, j = 9.0 Hz, 1H, 5′-H), 6.46 (d, j = 2.0 Hz, 1H, 8-H, 8-H,), 6.24 (d, j = 2.0 Hz, 1H, 6-H), 4.65 (S, 2H, COCH 2 ), 4.30 (q, j = 7.1 Hz, 2H, CH 3 CH 2 ), 1.32 (t, j = 7.1 Hz, 3H, CH 3 ).
[0016] 13 , 71.67, 60.95, 14.68.
Embodiment 2
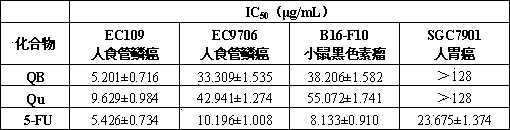
[0017] Example 2 Anti -tumor activity test
[0018] 2.1 Cell strain and reagent
[0019] Human esophageal squamous cell carcinoma cell EC109, human esophageal squamous cell carcinoma cell EC9706, human gastric cancer cell SGC7901 and mouse melanoma cell B16-F10, purchased at the cell library of the Shanghai Institute of Life Sciences of the Chinese Academy of Sciences.
[0020] Two Kobeya (DMSO), Sigma Company
[0021] Sigma Company
[0022] RPMI-1640 medium, Saimo Fei Shil Biochemical Products (Beijing) Co., Ltd.
[0023] Fetal Bull Serum, Simerfei Shier Biochemical Products (Beijing) Co., Ltd.
[0024] Fasterase, Hangzhou Gino Biomedical Technology Co., Ltd.
[0025] Cultivation base: Take the RPMI-1640 medium, add 10%fetal beef serum, penicillin 100U / ml, rinsein 100 μg / ml, mix well, and store for later use at 4 ° C.
[0026] Cell frozen liquid: Add DMSO to the cultivation base of 10%fetal cattle serum, so that the DMSO concentration is 10%, and it is now available.
[0027] Cor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com