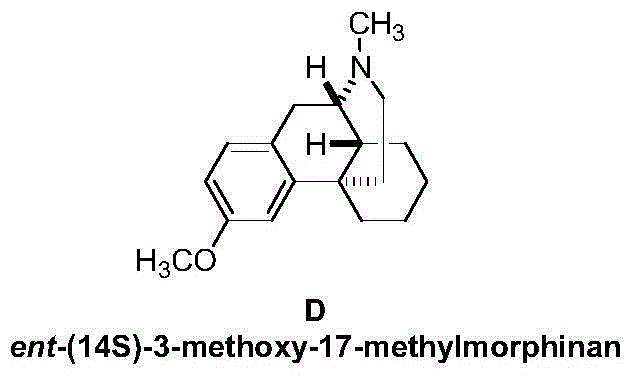
Preparation method of ent-(14S)-3-methoxyl-17-morphinan
A technology of methylmorphan and methoxy, which is applied in the field of preparation of ent--3-methoxy-17-methylmorphan, can solve the impurity ent-(14S)-3 which has not been specifically reported for dextromethorphan -Methoxy-17-methylmorphan preparation method, difficulties and other issues, to achieve the effect of high purity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] How to prepare ent-(14S)-3-methoxy-17-methylmorphan (D) simply and conveniently using the above-mentioned technical solution will be described below through a specific preparation process and method.
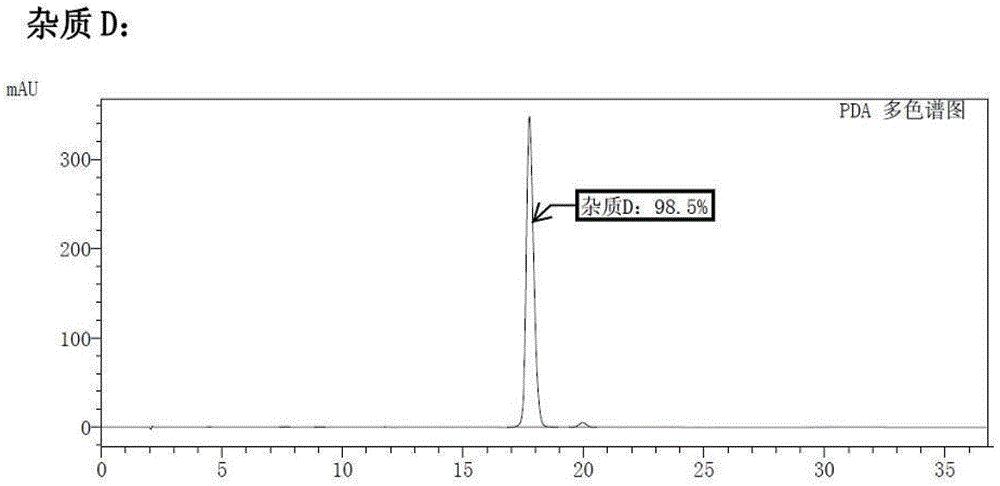
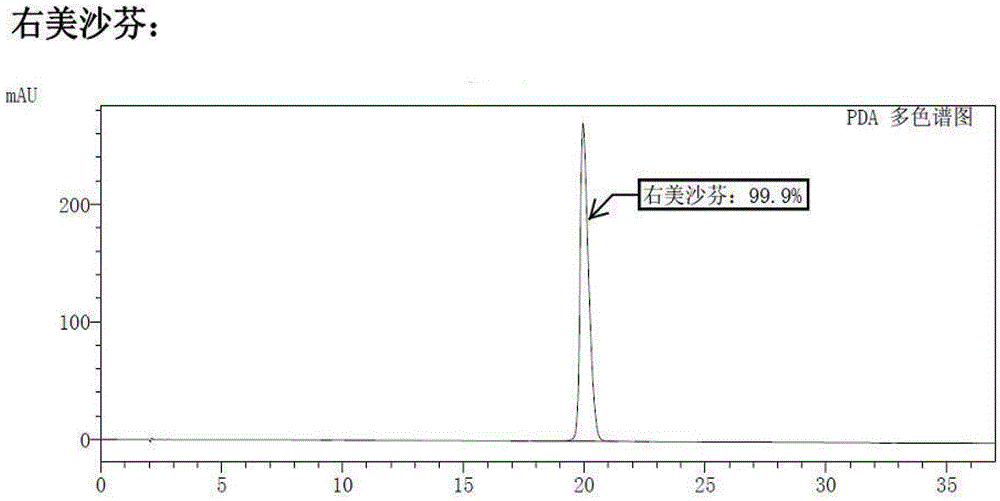
[0023] Add (+)-N-methyl-1-(4-methoxy)benzyl-1,2,3,4,5,6,7,8-octahydro Isoquinoline (I) (2.7g, 10mmol), dichloromethane 25mL, start stirring, and add aluminum trichloride (3.3g, 25mmol) in batches. Slowly raise the temperature to 40°C, vigorously stir the reaction for 5 hours, TLC detection, the reaction is complete. The solid was removed by filtration, washed successively with sodium bicarbonate solution and water, the organic phases were combined, dried and concentrated under reduced pressure to obtain a pale yellow oil ent-(14S)-3-methoxy-17-methylmorphan (D) 2.4 g, yield 88.6%. The purity was 98.5% by high performance liquid chromatography (HPLC). The chromatographic conditions are: Waters symmetry C18 / 280 nm / 25°C / acetonitrile:water=60:40 (containing docusate sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com