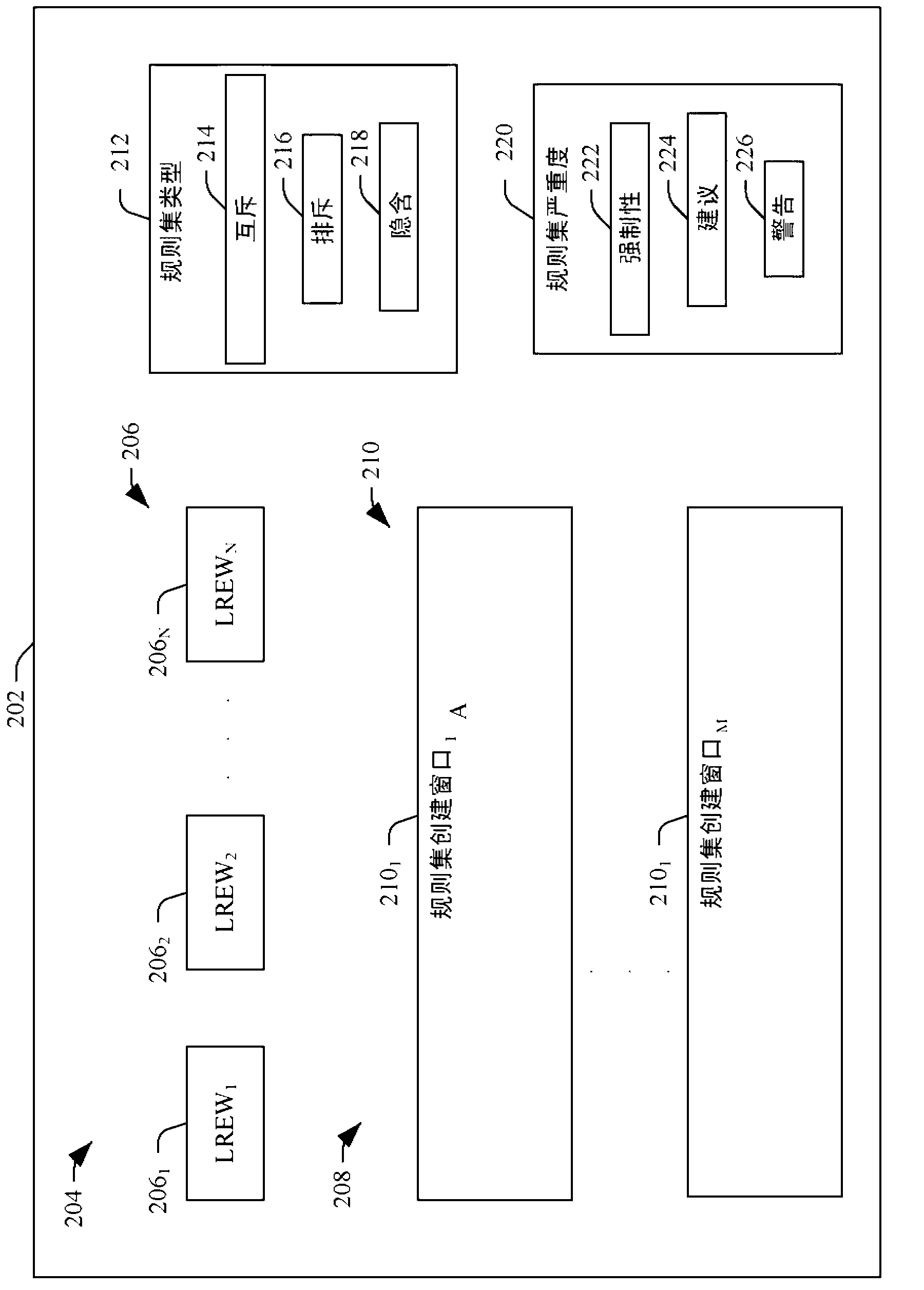
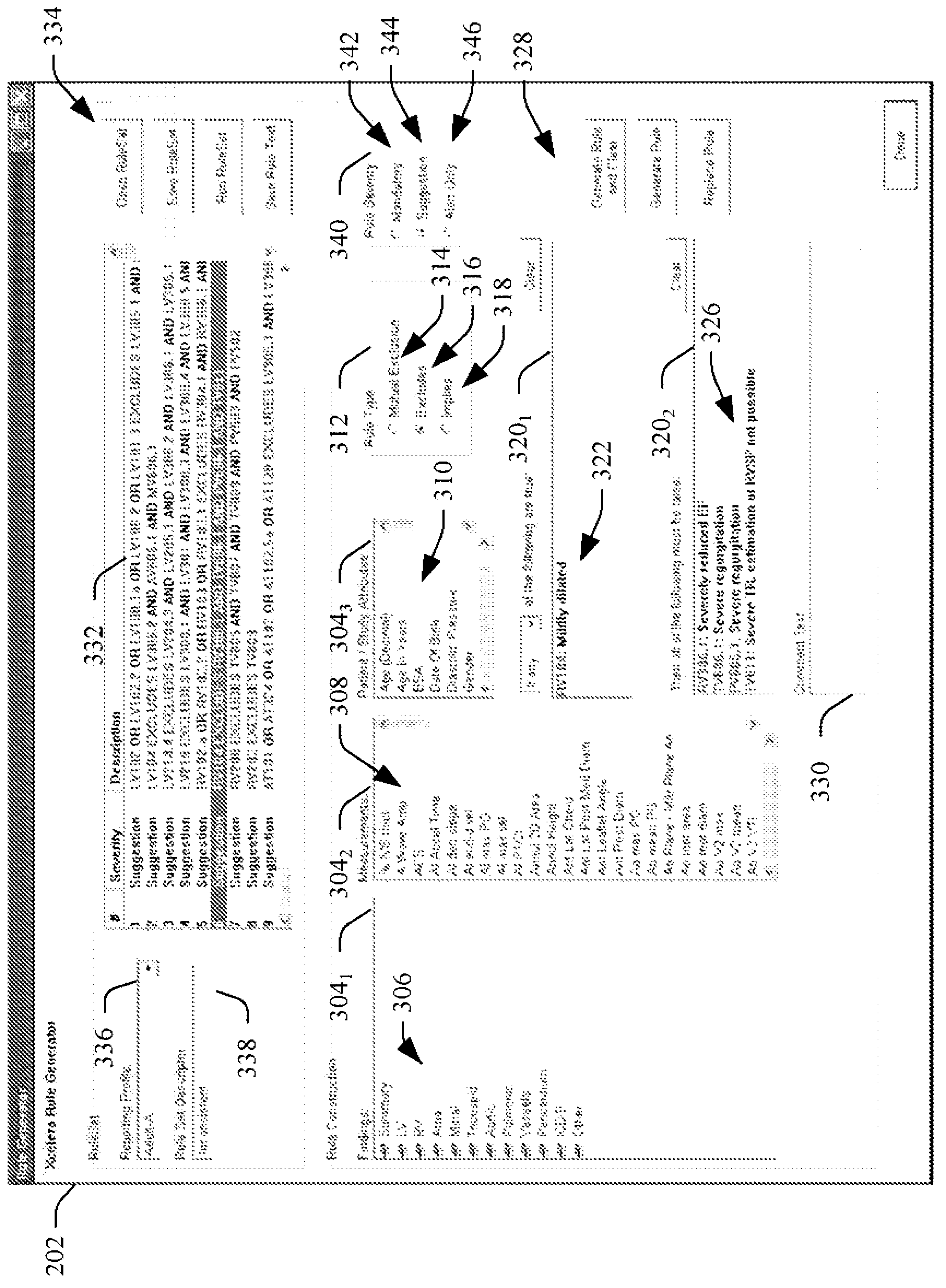
Medical information system ruleset creation and/or evaluation graphical user interface
A technology of graphical user interface and rules, applied in the field of medical information system, can solve problems such as the difficulty of CDS system algorithm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
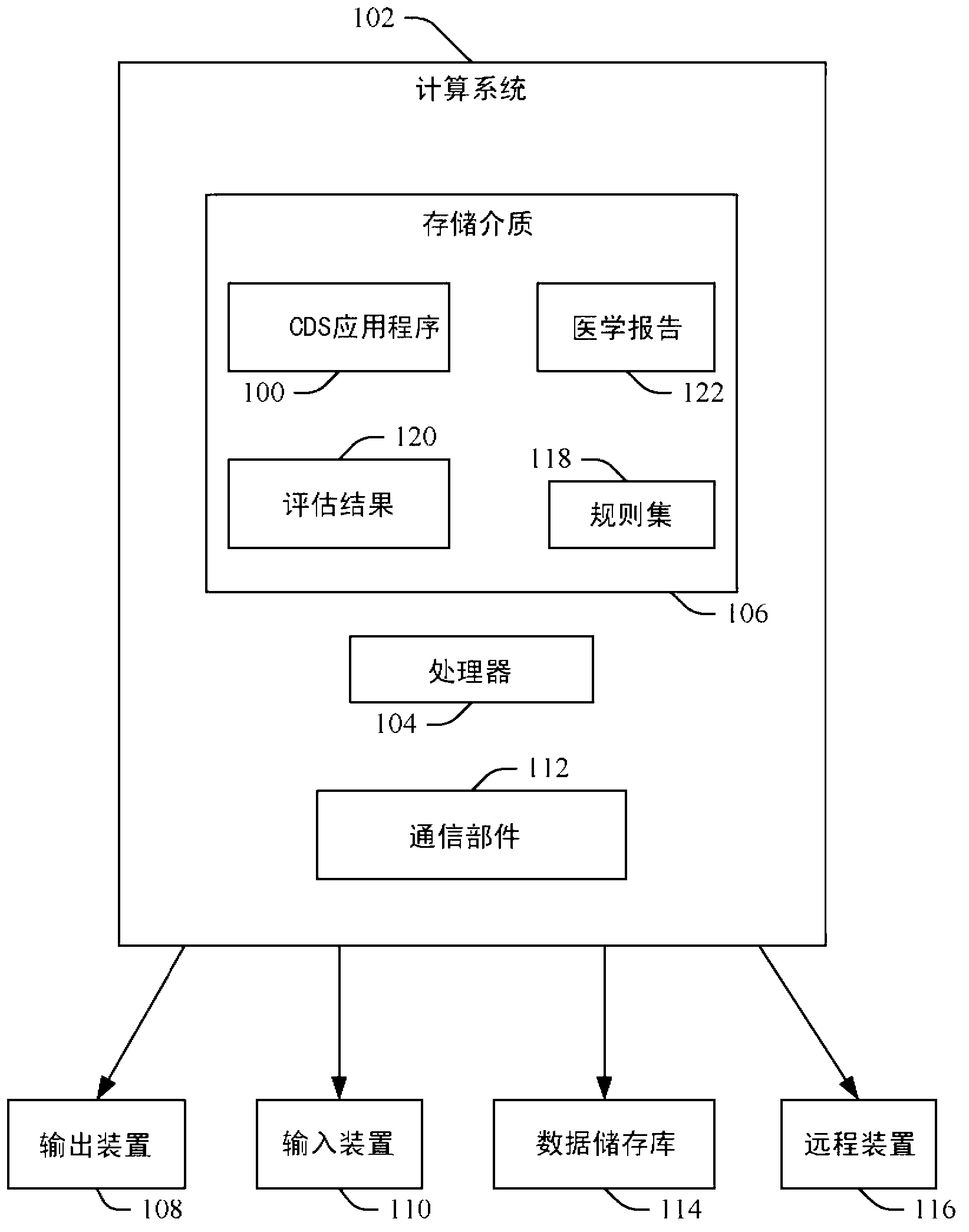
[0020] figure 1 An exemplary computing system 102 such as a workstation, computer, etc. is shown. Computing system 102 includes one or more processors 104 and a computer-readable storage medium 106 (physical memory) encoded with or embedded with computer-readable instructions that, when executed by one or more processors 104, time causes the system 102 to perform various functions. The computing system 102 also includes one or more output devices 108, such as displays, printers, etc., that can be used for visual presentation and / or providing information.
[0021] Computing system 102 also includes one or more input devices 110 , such as a keyboard, keypad, mouse, trackball, digital pen, microphone, etc., that allow a user to interact with computing system 102 . Computing system 102 also includes a communication component 112 that allows system 102 to communicate with one or more data repositories 114, such as a picture archiving and communication system (PACS), The remote d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com