Dehydrogenation method
A technology of dehydrogenation and dehydrogenation catalyst, applied in the field of dehydrogenation of cyclohexane and methylcyclopentane, which can solve the problem of low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Dehydrogenation of Cyclohexane
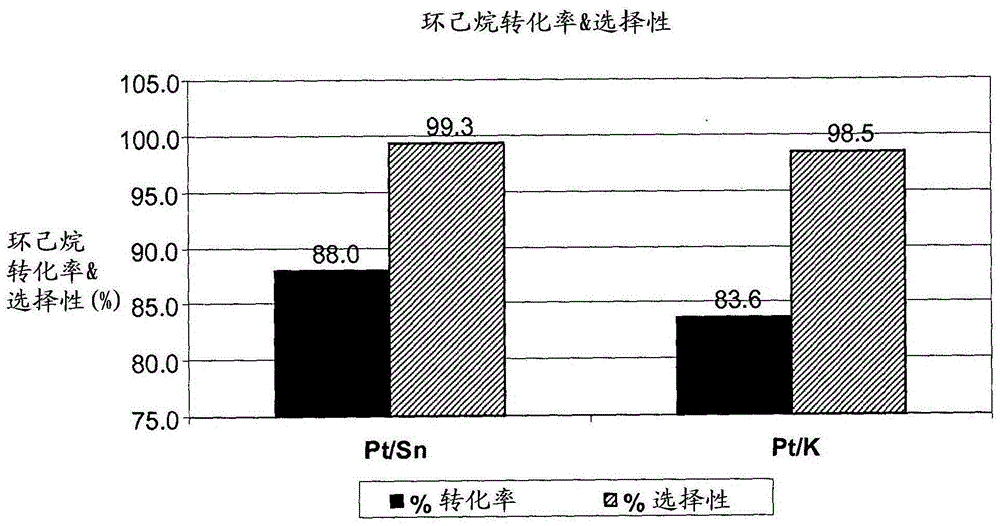
[0069] A first dehydrogenation catalyst comprising 1 wt% platinum and 0.15 wt% tin on a silica support and a second dehydrogenation catalyst (comparative) comprising 1 wt% platinum and 1 wt% potassium on a silica support were crushed to 60 / 100 mesh and loaded into a 1 / 2" (1.27cm) outer diameter (OD) tubular downflow reactor. The catalyst was then separately mixed with 89 wt% benzene, 10 wt% cyclohexane and 1 wt% methylcyclo Composition of pentane at 480°C, 0.689MPa, 10hr -1 Weight hourly space velocity (WHSV) and 4 hydrogen and hydrocarbon (H 2 / HC) molar ratio of dehydrogenation conditions. Such as figure 1 As shown, the Pt / Sn / SiO 2 Catalyst and the Pt / K / SiO 2 Catalysts achieve significantly higher conversions and selectivities of cyclohexane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com