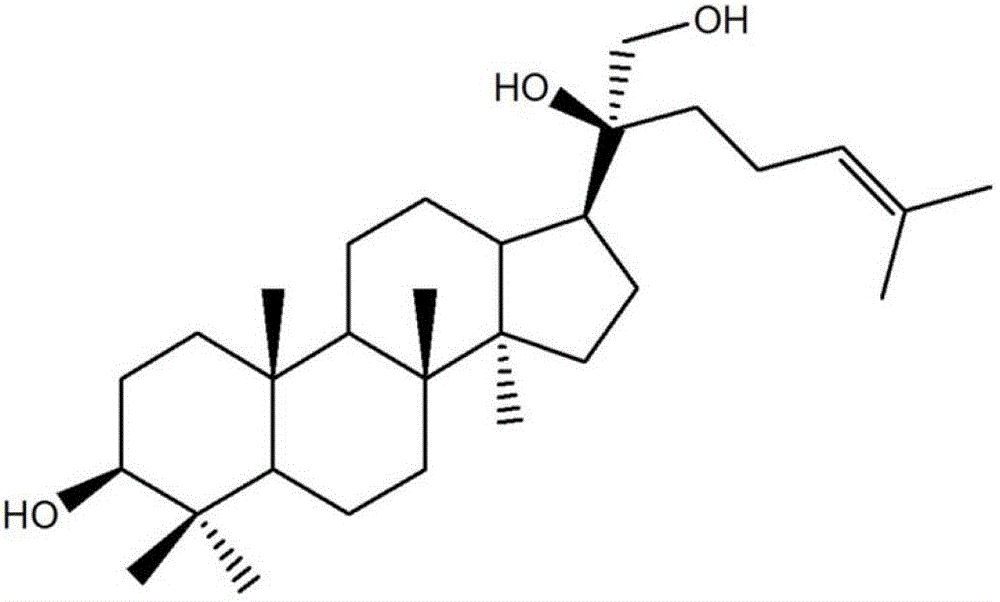
Use of 3β,20(s),21-trihydroxydammarane-24-ene in the preparation of tumor multidrug resistance reversal agent
A technology of trihydroxydammarane and drug resistance reversal, applied in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of affecting the pharmacokinetics of anticancer drugs, not getting the expected effect, restricting the scope of application, etc. Achieve the effect of overcoming drug resistance, inhibiting proliferation, and enhancing therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: CCK-8 kit detects the cytotoxicity of H6
[0037] Experimental Materials:
[0038] H6 is extracted from Gynostemma pentaphyllum, a plant of Cucurbitaceae, with a purity of not less than 95%. Human liver cancer cell line HepG2, human liver cancer cell line SMMC-7721, human liver cancer cell line Hep3B, human leukemia cell line THP-1, human leukemia cell line K562, undifferentiated human gastric cancer cell line HGC-27, human ovarian cancer cell line SKOV3, human pancreatic cancer cell line PANC-1, human colon cancer cell line SW480, human cervical cancer cell line HeLa, human lung adenocarcinoma cell line A549, and human breast cancer cell line MDA-MB-453 were purchased from Shanghai Biochemical Cell, Chinese Academy of Sciences cell bank. Human umbilical vein epithelial cells (HUVEC-2C) cell lines were purchased from Cascade Biologics Biotechnology Company.
[0039] experimental method:
[0040] Cell recovery
[0041] 1) Take out the cryotube from the li...
Embodiment 2
[0063] Example 2: CCK-8 kit detects drug-resistant cell lines' resistance to H6
[0064] Experimental Materials:
[0065] Verapamil (VPL), vincristine (VCR) and doxorubicin (ADR) were purchased from Roche Chemical Company with a purity greater than 99%. CCK-8 kit was purchased from Tongren Company.
[0066] Human oral epithelial carcinoma KB cells and their vincristine-resistant strain KB / VCR, human breast cancer MCF-7 cells and their adriamycin-resistant strain MCF-7 / ADR were prepared by Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
[0067] experimental method:
[0068] The methods of cell recovery and cell cryopreservation are the same as in Example 1.
[0069] Subculture of cells: Human oral epithelial carcinoma KB cells and their vincristine-resistant strain KB / VCR were cultured in α-MEM medium containing 10% FBS, 2mM glutamine and 1mM sodium pyruvate. Adriamycin-resistant human breast cancer cell line MCF-7 / ADR was cultured in α-MEM medium contai...
Embodiment 3
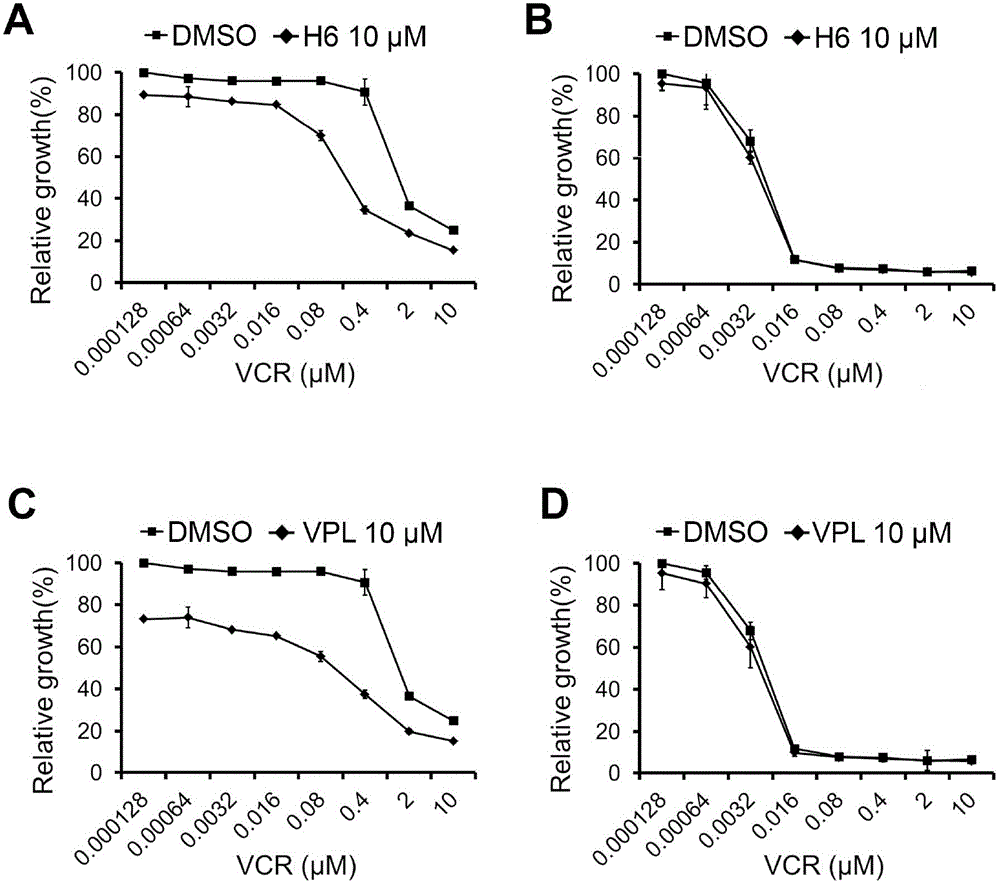
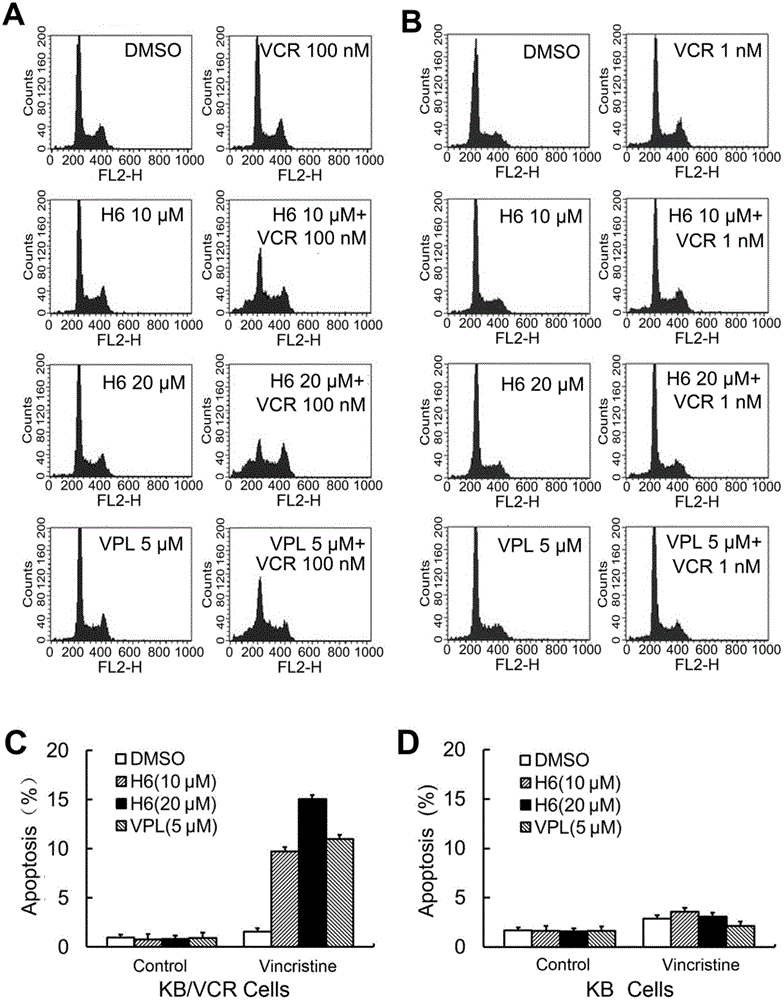
[0079] Example 3: The CCK-8 method detects the reversal effect of H6 on the multidrug tolerance activity of drug-resistant cell lines
[0080] Experimental material: with embodiment 2.
[0081] experimental method:
[0082] H6 sensitization experiment: KB and KB / VCR cells were inoculated into 96-well plates at a density of 3500 / well, and after 24 hours, different concentrations of VCR, and different concentrations of VCR and H6 were mixed with α-MEM containing 10% fetal bovine serum After being prepared, it is added to each hole. After culturing for 48 hours, discard the culture medium, and use the CCK-8 kit to measure the activity of drug-resistant cell lines and their parental cells to VCR and VCR+H6 in the same manner as in Example 1, and draw a curve.
[0083] Three replicate wells were set up for each concentration, and the experiment was repeated three times.
[0084] Experimental results:
[0085] The multiplicity of drug resistance of KB / VCR cells to VCR was 81.9 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com