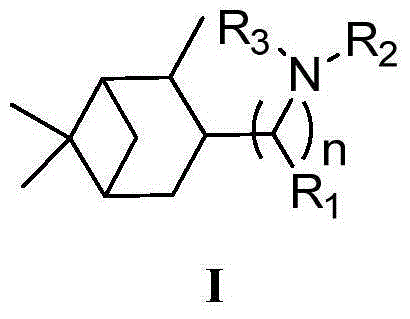
Novel naphthenic amine compounds for anti-mutant influenza virus
一种流感病毒、化合物的技术,应用在新型环烷胺类化合物领域,能够解决毒副作用、没有新型结构的药物、易产生耐药等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the general synthetic procedure of compound 1-10:
[0043] in CH 3 After dissolving (1R,2R,3R,5S)-(□)-pinamine (1g, 6.5mmol) in OH (20mL), add aldehyde or ketone (1.5equiv., 9.8mmol), and stir the reaction solution at room temperature for 1h , followed by NaBH(OAc) 3 (5.5g, 26mmol), after adding 1 drop of acetic acid dropwise, the reaction was stirred for 10h. Then, saturated sodium bicarbonate solution was added to quench the reaction, extracted with dichloromethane (3x20ml), the combined organic phases were washed twice with saturated brine, and dried over anhydrous sodium sulfate. The oil obtained by column chromatography was washed with HCl / CH 3 After treatment with OH (20 mL), evaporation to dryness resulted in a solid that was washed with ether (3x20 mL) to give the title compound.
Embodiment 2
[0044] Example 2: (1R,2R,3R,5S)-2,6,6-trimethyl-N-((5-methyl-1H-4-imidazolyl)methyl)bicyclo[3.1.1] Heptane-3-amine (Compound 1)
[0045]
[0046] 1 HNMR (400MHz, DMSO-d 6 )δ0.92(s,3H),1.17(d,3H,J=7.2Hz),1.19(s,3H),1.45(d,2H,J=9.6Hz),1.78(s,1H),1.95( s,2H),2.02 2.07(m,1H),2.19 2.27(m,2H),2.34(s,1H),2.38(s,3H),3.53(s,1H),4.28(s,2H),9.08 (s,1H),9.54(s,1H),10.10(s,1H),14.90(br,2H); 13 CNMR (125MHz, DMSO-d 6 )δ9.13, 20.75, 23.26, 27.23, 30.55, 31.72, 37.13, 38.38, 40.00, 40.28, 46.97, 55.96, 119.91, 129.79, 133.16; HRMS: calculated for C 15 h 25 N 3 (M+H + ):248.38,found:248.2121.
Embodiment 3
[0047] Example 3: (1R,2R,3R,5S)-2,6,6-trimethyl-N-((5-cyclopropyl-1H-4-imidazolyl)methyl)bicyclo[3.1.1 ]heptane-3-amine (compound 2)
[0048]
[0049] 1 HNMR (400MHz, DMSO-d 6 )δ0.92(s,3H),1.01(d,2H,J=8.8Hz),1.12(s,3H,J=7.2),1.17 1.20(m,6H),1.75 1.81(m,2H),1.90 1.97(m,2H),2.00 2.07(m,3H),3.53(s,1H),4.36(s,2H),8.27(br,1H),9.03(d,1H,J=8),9.57(br ,1H),10.13(br,1H),14.66(br,1H);ESI-MS:calculatedforC 17 h 27 N 3 (M+H + ):274.42,found:274.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com