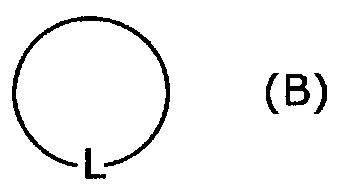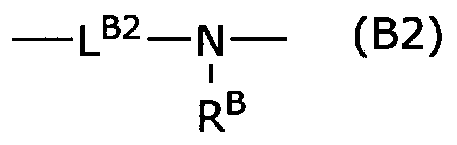Patents
Literature
90 results about "Cycloalternan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for making lubricating base oils with high ratio of monocycloparaffins to multicycloparaffins
InactiveUS20060289337A1Liquid hydrocarbon mixture productionTreatment with hydrotreatment processesMolecular sieveWax
A process for manufacturing a lubricating base oil, comprising dewaxing a substantially paraffinic wax feed by hydroisomerization dewaxing using a shape selective intermediate pore size molecular sieve under hydroisomerization conditions including a hydrogen to feed ratio from about 712.4 to about 3562 liter H2 / liter oil, whereby a lubricating base oil is produced having a)a total weight percent of molecules with cycloparaffinic functionality greater than 10, and b) a ratio of weight percent molecules with monocycloparaffinic functionality to weight percent molecules with multicycloparaffinic functionality greater than 30. Also a method for producing a base oil having a high ratio of weight percent molecules with monocycloparaffinic functionality to weight percent molecules with multicycloparaffinic functionality by hydroisomerization dewaxing a selected Fischer-Tropsch wax under hydroisomerization conditions including a hydrogen to feed ratio from about 712.4 to about 3562 liter H2 / liter oil. Also a lubricating base oil manufacturing plant.
Owner:CHEVROU USA INC
Lower ash lubricating oil with low cold cranking simulator viscosity
A lubricating oil comprising: a) at least 5 wt % of lubricating base oil, made from a waxy feed, having >10 wt % molecules with cycloparaffinic functionality, a ratio of molecules with monocycloparaffinic functionality to molecules with multicycloparaffinic functionality >20, and b) a DI additive package; wherein the lubricating oil contains <0.2 wt % VI improver and wherein the lubricating oil has a low sulfated ash and a low CCS viscosity at −20° C. A lubricating oil with a kinematic viscosity at 100° C. between 12.5 and 16.3 cSt with a low CCS viscosity comprising: a) a lubricating base oil, made with a waxy feed, having a viscosity index >150, b) up to 75 wt % unconventional petroleum derived bright stock, c) a lower ash DI additive package, and d) <0.2 wt % viscosity index improver. A process to make lubricating oil with a low sulfated ash and low CCS viscosity.
Owner:CHEVROU USA INC
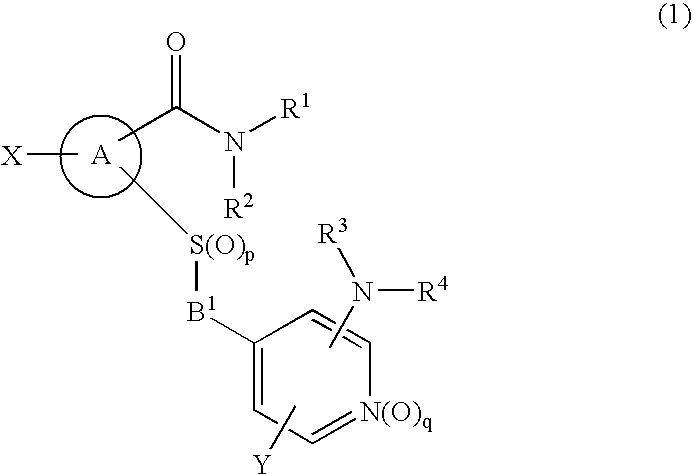
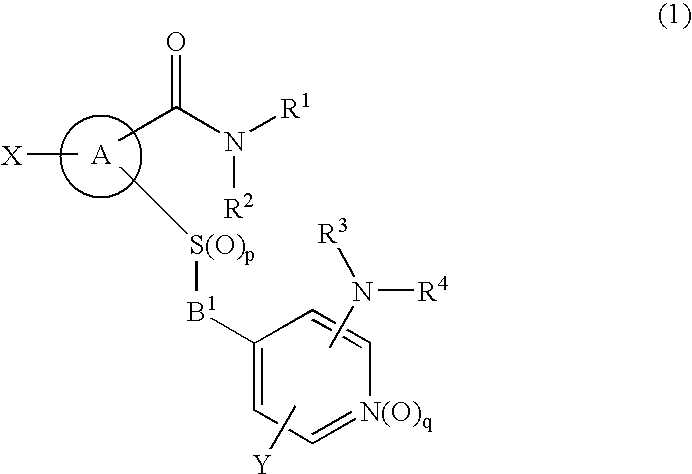
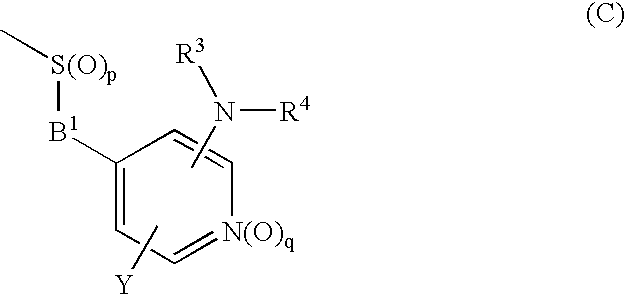
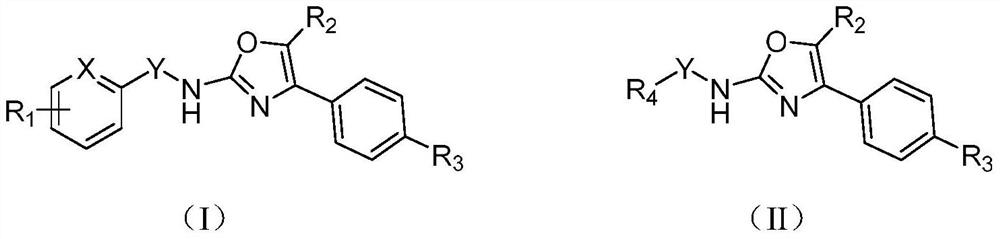
Novel cyclic compound having 4-pyridylalkylthio group having substituted or unsubstituted amino group introduced therein
InactiveUS20070149574A1Useful in therapyExcellent cell proliferation inhibitory effectBiocideSenses disorderDiseaseAryl
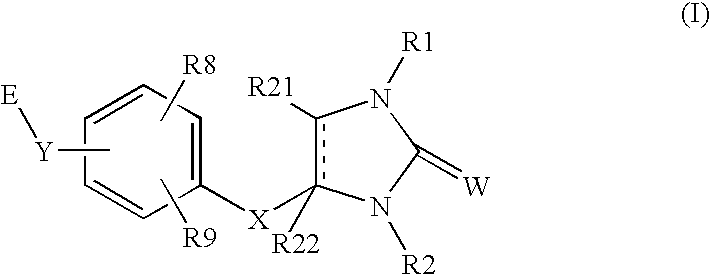
A novel cyclic compound having a 4-pyridylalkylthio group having an (un)substituted amino group introduced therein or a salt thereof. They are useful as a medicine. The cyclic compound is a compound represented by the following formula (1), which is useful for the treatment of diseases in which angiogenesis participates. In the following formula (1), ring A represents a benzene ring or a 5- or 6-membered aromatic heterocycle optionally fused with a cycloalkane ring; B represents alkylene; R1 and R2 each represents H, (substituted) aryl, (substituted) heterocyclic group, etc.; R3 and R4 each represents H, (substituted) alkyl, (substituted) cycloalkyl, -Z-R5, etc.; R5 represents (substituted) alkyl, (substituted) aryl, (substituted) heterocyclic group, etc.; X and Y each represents H, etc.; Z represents —CO—, —COO—, —CONR6—, —SO2—, etc.; R6 represents H, etc.; p is 0, 1, or 2; and q is 0 or 1.
Owner:SANTEN PHARMA CO LTD
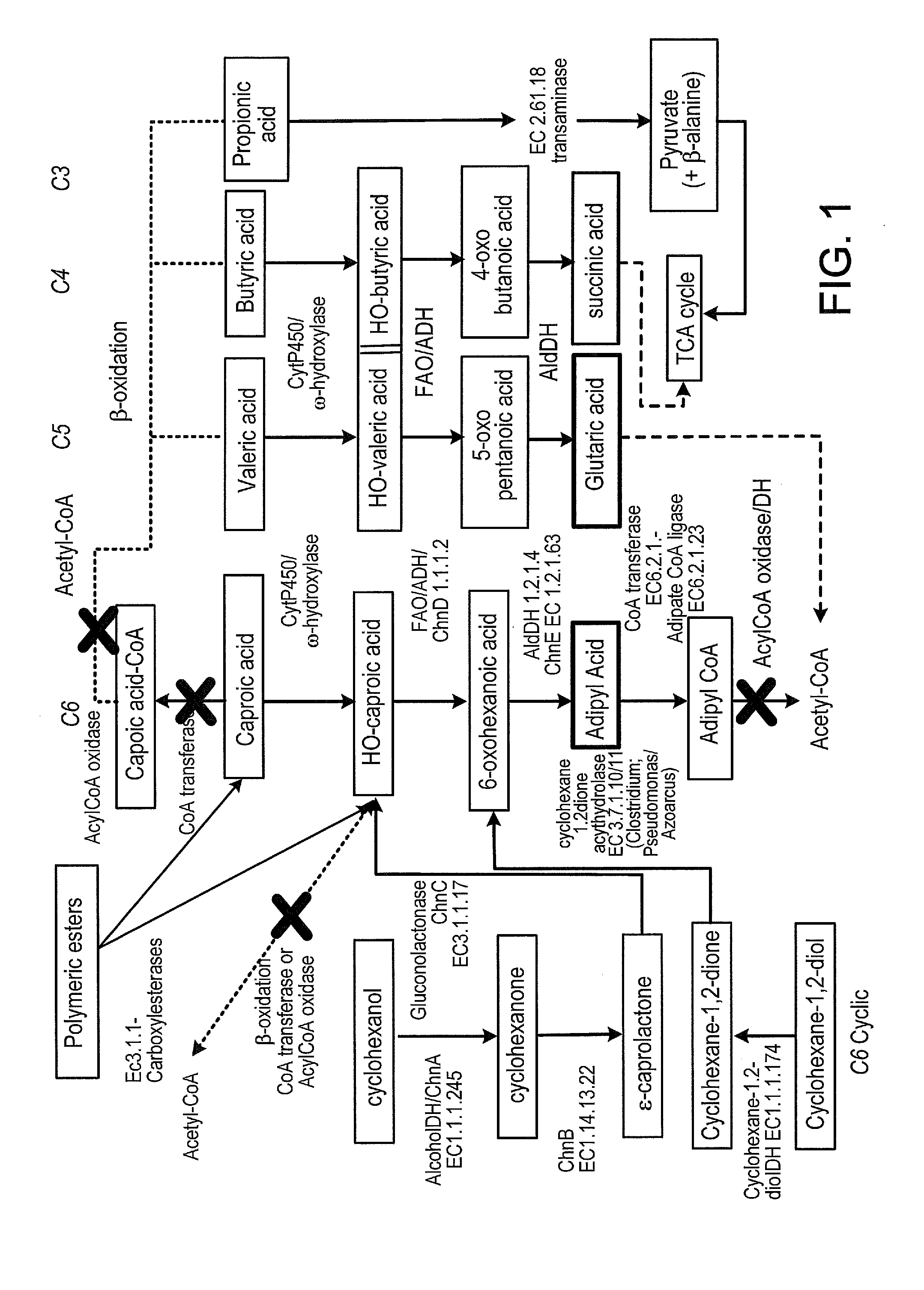
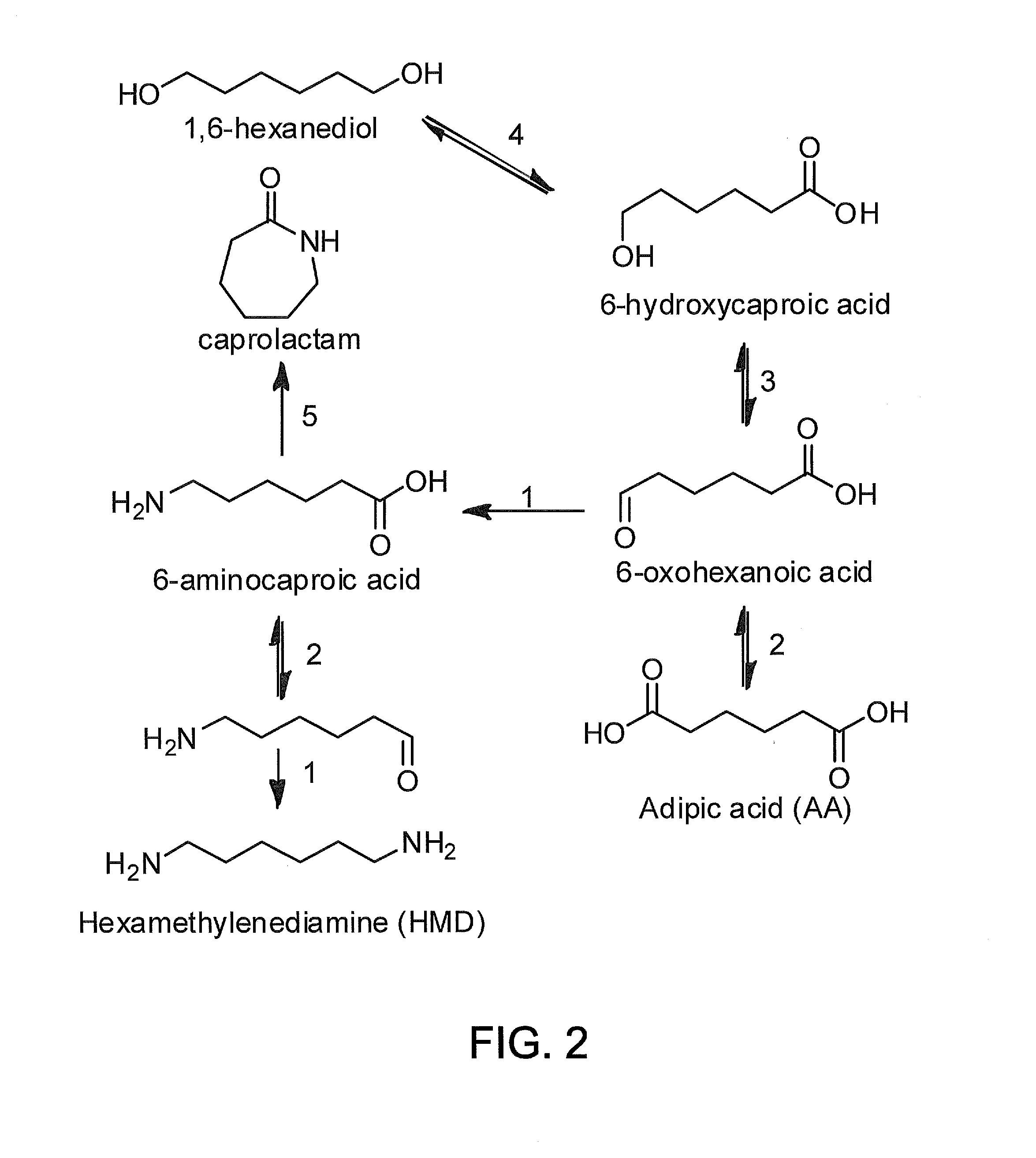
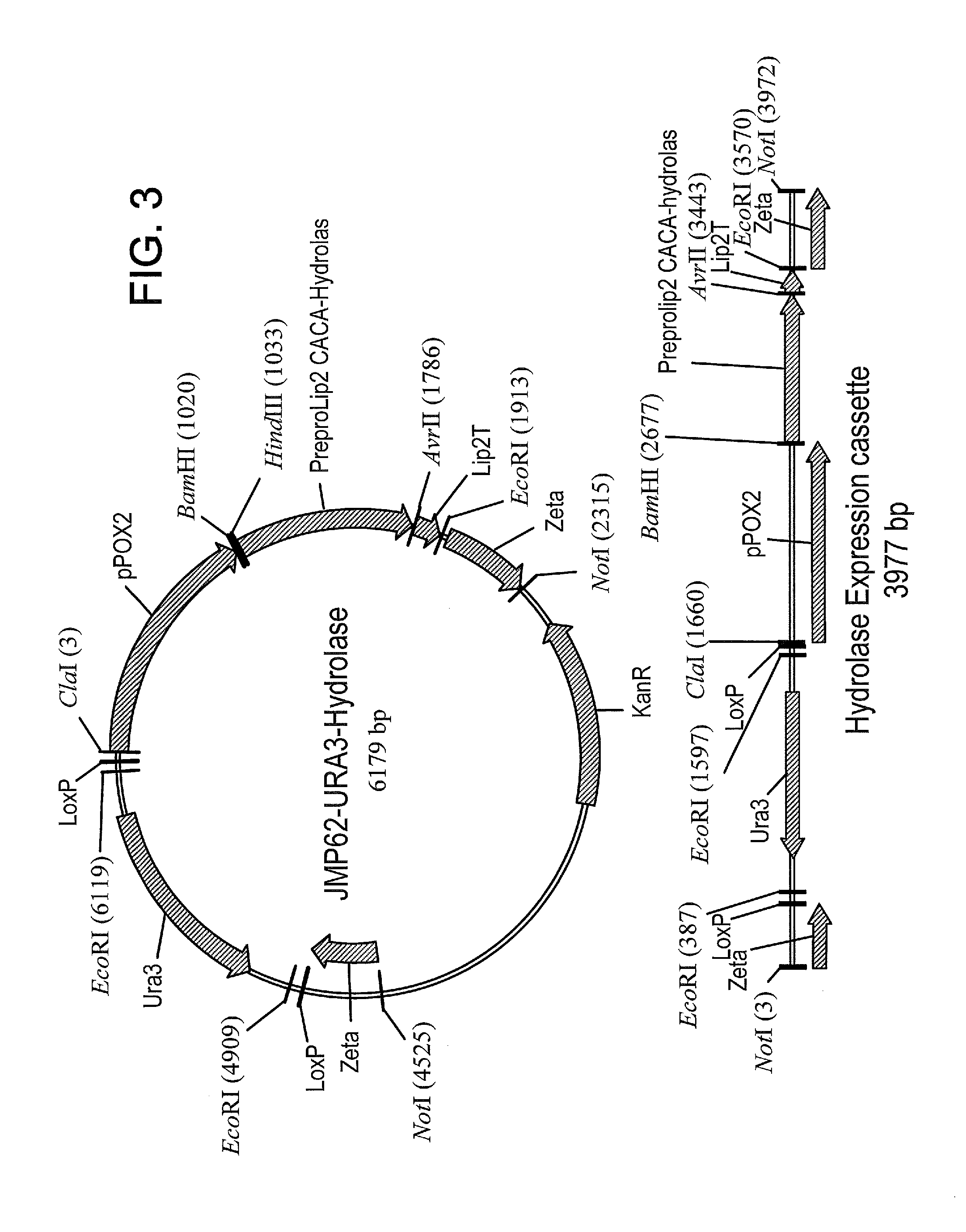
Methods of producing carboxylic acids
The invention relates to methods for enriching monomer content in a cycloalkane oxidation process mixed organic waste stream. In particular, the methods involve combining a biocatalyst with a mixed organic waste stream from a cycloalkane oxidation process, and enzymatically converting dimeric and / or oligomeric components of said waste stream into monomeric components. The methods may enrich the content of diacids, adipic acid, and / or other α,ω-difunctional C6 alkanes in the mixed organic waste stream. Additionally, the treated mixed organic waste streams may have improved burning efficiency.
Owner:INV NYLON CHEM AMERICAS LLC
Process for preparing cycloalkanone
InactiveUS6528658B1Efficient productionIncrease conversionsPreparation by oxidation reactionsOrganic compound preparationImideBoiling point
A series of steps of (A) a step for bringing a cycloalkane into contact with molecular oxygen (oxidizing reactor 2) in the presence of an oxidizing catalyst having an imide unit of the following formula (I):wherein X represents oxygen atom or hydroxyl group;(B) a step for separating the catalyst, and by-produced acid component or a derivative thereof from the reaction mixture (filter 3, extracting column 4, hydrolyzing unit 7, saponifying unit 8); and(C) steps for separating the cycloalkane, a cycloalkanol, and a cycloalkanone from the reaction mixture individually (distilling columns 5, 6, 9, and 10) makes it possible to produce cycloalkanones efficiently. A first component (lower-boiling point component) containing the cycloalkane and a second component (higher-boiling point component) containing the cycloalkanone and cycloalkanol may be separated from the reaction mixture, and the cycloalkanone and the cycloalkanol may be separated from the higher-boiling point component. Such production process is capable of providing cycloalkanones with high efficiency.
Owner:DAICEL CHEM IND LTD
Heterocyclo-substituted imidazoles for the treatment of inflammation
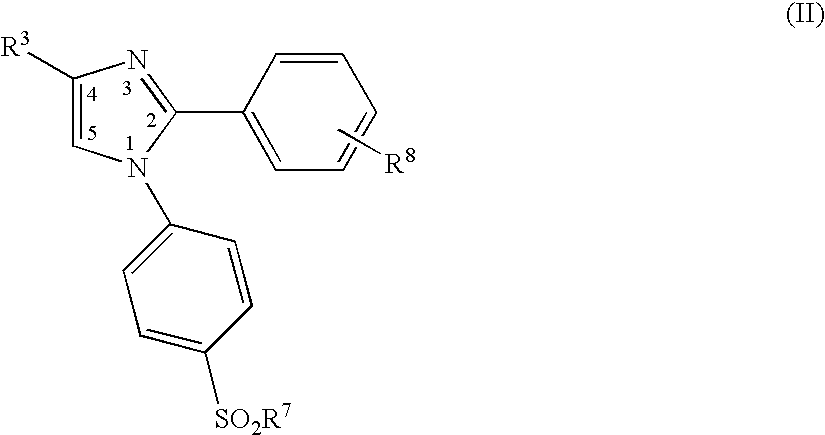
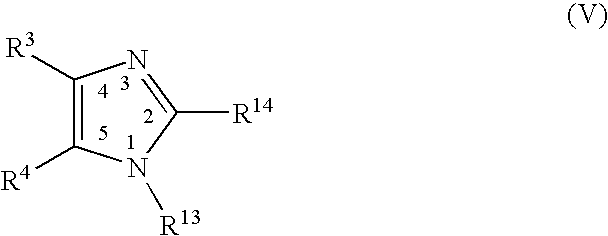
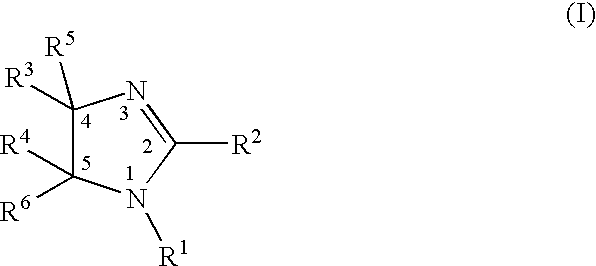
A class of imidazolyl compounds is described for use in treating inflammation. Compounds of particular interest are defined by formula (V), wherein R3 is a radical selected from hydrido, alkyl, haloalkyl, aralkyl, heterocycloalkyl, heteroaralkyl, acyl, cyano, alkoxy, alkylthio, alkylthioalkyl, alkylsulfonyl, cycloalkylthio, cycloalkylthioalkyl, cycloalkylsulfonyl, cycloalkylsulfonylalkyl, haloalkylsulfonyl, arylsulfonyl, halo, hydroxyalkyl, alkoxyalkyl, alkylcarbonyl, arylcarbonyl, aralkylcarbonyl, heterocyclocarbonyl, cyanoalkyl, aminoalkyl, alkylaminoalkyl, N-arylaminoalkyl, N-alkyl-N-arylaminoalkyl, carboxyalkyl, alkoxy-carbonylalkyl, alkoxycarbonyl, haloalkylcarbonyl, carboxyl, aminocarbonyl, alkylaminocarbonyl, alkylaminocarbonylalkyl, heteroarylalkoxyalkyl, heteroaryloxyalkyl, heteroarylthioalkyl, aralkoxy, aralkylthio, heteroaralkoxy, heteroaralkylthio, heteroarylalkylthioalkyl, heteroaryloxy, heteroarylthio, arylthioalkyl, aryloxyalkyl, arylthio, aryloxy, aralkylthioalkyl, aralkoxyalkyl, aryl and heteroaryl; wherein R4 is a radical selected from hydrido, alkyl and halo; and wherein R13 and R14 are independently selected from aryl and heterocyclo, wherein R13 and R14 are optionally substituted at a substitutable position with one or more radicals independently selected from alkylsulfonyl, aminosulfonyl, halo, alkylthio, alkyl, cyano, carboxyl, alkoxycarbonyl, haloalkyl, hydroxyl, alkoxy, hydroxyalkyl, alkoxyalkyl, haloalkoxy, amino, alkylamino, arylamino and nitro; provided at least one of R13 and R14 is aryl substituted with alkylsulfonyl or aminosulfonyl; or a pharmaceutically-acceptable salt thereof
Owner:KHANNA ISH K +7
Heterocyclo-substituted imidazoles for the treatment of inflammation
A class of imidazolyl compounds is described for use in treating inflammation. Compounds of particular interest are defined by formula (V), wherein R3 is a radical selected from hydrido, alkyl, haloalkyl, aralkyl, heterocycloalkyl, heteroaralkyl, acyl, cyano, alkoxy, alkylthio, alkylthioalkyl, alkylsulfonyl, cycloalkylthio, cycloalkylthioalkyl, cycloalkylsulfonyl, cycloalkylsulfonylalkyl, haloalkylsulfonyl, arylsulfonyl, halo, hydroxyalkyl, alkoxyalkyl, alkylcarbonyl, arylcarbonyl, aralkylcarbonyl, heterocyclocarbonyl, cyanoalkyl, aminoalkyl, alkylaminoalkyl, N-arylaminoalkyl, N-alkyl-N-arylaminoalkyl, carboxyalkyl, alkoxycarbonylalkyl, alkoxycarbonyl, haloalkylcarbonyl, carboxyl, aminocarbonyl, alkylaminocarbonyl, alkylaminocarbonylalkyl, heteroarylalkoxyalkyl, heteroaryloxyalkyl, heteroarylthioalkyl, aralkoxy, aralkylthio, heteroaralkoxy, heteroaralkylthio, heteroarylalkylthioalkyl, heteroaryloxy, heteroarylthio, arylthioalkyl, aryloxyalkyl, arylthio, aryloxy. aralkylthioalkyl, aralkoxyalkyl, aryl and heteroaryl; wherein R4 is a radical selected from hydrido, alkyl and halo; and wherein R13 and R14 are independently selected from aryl and heterocyclo, wherein R13 and R14 are optionally substituted at a substitutable position with one or more radicals independently selected from alkylsulfonyl, aminosulfonyl, halo, alkylthio, alkyl, cyano, carboxyl, alkoxycarbonyl, haloalkyl, hydroxyl, alkoxy, hydroxyalkyl, alkoxyalkyl, haloalkoxy, amino, alkylamino, arylamino and nitro; provided at least one of R13 and R14 is aryl substituted with alkylsulfonyl or aminosulfonyl; or a pharmaceutically-acceptable salt thereof.
Owner:GD SEARLE & CO
Cycloalkyl and heterocycloalkyl inhibitors as well as preparation method and application thereof
PendingCN112694475AGood selective inhibitionGood pharmacodynamicsOrganic active ingredientsOrganic chemistrySide effectCombinatorial chemistry
Owner:SUZHOU ZELGEN BIOPHARML +1
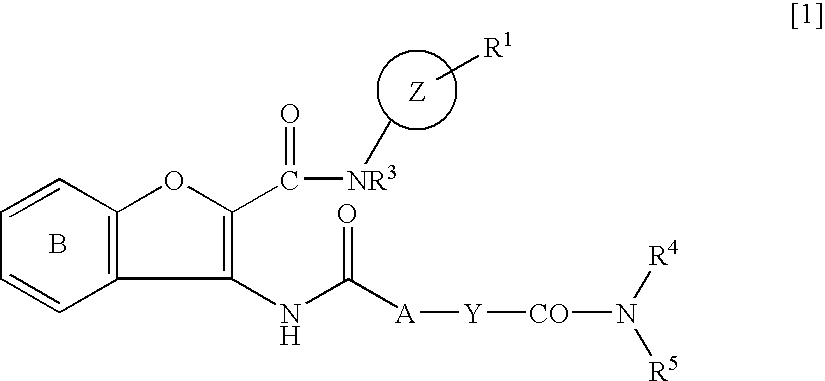
Carbamoyl-type benzofuran derivatives
The present invention provides a carbamoyl-type benzofuran derivative of the formula [1]: wherein Ring Z is a group of the formula: etc.; A is a single bond, and the like; Y is a cycloalkanediyl group, etc.; R4 and R5 are the same or different and each is an optionally substituted lower alkyl group, etc.; R1 is a halogen atom, etc.; Ring B of the formula: is an optionally substituted benzene ring; and R3 is a hydrogen atom. etc., or a pharmaceutically acceptable salt thereof, which is useful as an FXa inhibitor.
Owner:MITSUBISHI TANABE PHARMA CORP
Production method of rubber filling oil excellent in stability
ActiveCN104560172AImprove stabilityFix stability issuesTreatment with hydrotreatment processesPolycyclic aromatic hydrocarbonDistilled oil
The invention relates to a production method of rubber filling oil excellent in stability. The production method comprises the following steps: sequentially charging decompression distilled oil of naphthenic crude oil and cycloalkane immediate crude oil, and light deasphalted oil into a hydro-treatment reaction area, a hydro-dewaxing reaction area and a hydro-refining reaction area; charging a hydro-refining product into an electromagnetic wave treatment area; removing a part of saturated polycyclic aromatic hydrocarbon under the irradiation of high-temperature electromagnetic wave; and charging a product which is obtained in the electromagnetic wave treatment area into a degumming unit to obtain a degummed fraction which is just the rubber filling oil product. The method disclosed by the invention has the advantages of improving the stability of the rubber filling oil without using noble metals for hydro-refining or adding additives, greatly simplifying the technological production procedures of the rubber filling oil and greatly reducing the device construction investment and the operation cost.
Owner:CHINA PETROLEUM & CHEM CORP +1
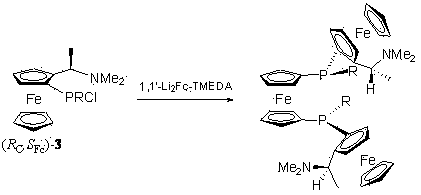
Chiral ferrocene diphosphine ligand and preparation method thereof
The invention provides a chiral ferrocene diphosphine ligand and a preparation method thereof, and discloses a compound as shown in a general structural formula (I), wherein R is selected from C1-C25 alkyl, aryl or substituted aryl, hetero-aryl and cycloalkyl; R' is selected from C1-C25 alkyl, aryl or substituted aryl, hetero-aryl and cycloalkyl. In the chiral ferrocene diphosphine ligand disclosed by the invention, various substituent groups can be conveniently introduced above phosphorus atoms, and electrons and three-dimensional properties of the phosphine ligand are easy to be changed, so that the application range of the phosphine ligand in different catalytic reactions can be farthest widened.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Hydraulic fluid compositions and preparation thereof
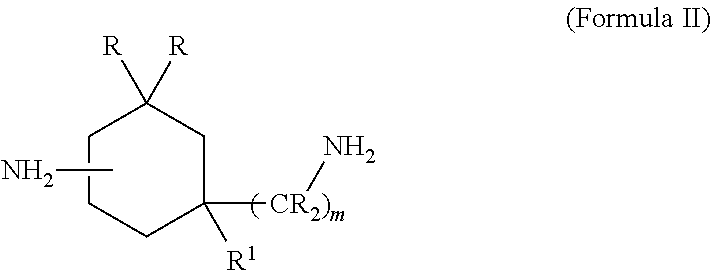
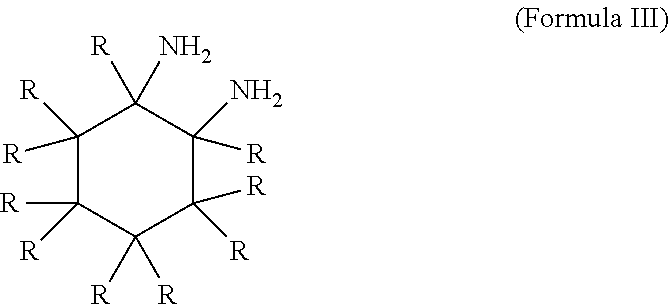
InactiveUS7674364B2Minimize deterioration and leakageAdditivesBase-materialsIsomerizationCycloalternan
A hydraulic fluid composition having excellent seal compatibility is prepared from an isomerized base oil is provided. The composition comprising (i) 80 to 99.999 wt. % of a lubricating base oil having consecutive numbers of carbon atoms, less than 10 wt % naphthenic carbon by n-d-M, less than 0.10 wt. % olefins and less than 0.05 wt. % aromatics, a molecular weight of greater than 600 by ASTM D 2503-92 (Reapproved 2002), a wt % total molecules with cycloparaffinic functionality greater than 25 and a ratio of molecules with monocycloparaffinic functionality to molecules with multicycloparaffinic functionality greater than 10; and (ii) optionally from 0.001 to 6 wt % of a viscosity modifier; and (iii) 0-10 wt % of at least an additive package. When used in operations, the composition results in an average volume change in a rubber seal of less than 3% and an average hardness change in the rubber seal of less than 1 pts. when tested under ASTM D 471-06 (SRE NBR1 at 100° C., 168 hours).
Owner:CHEVROU USA INC
Heterogeneous copper porphyrin as well as preparation method and application thereof
PendingCN110918122AA New Method for Efficient and Selective Catalytic OxidationFeasible new method of selective catalytic oxidationPreparation by oxidation reactionsOrganic compound preparationPtru catalystPorphyrin
Provided is heterogeneous copper porphyrin; a preparation method comprises the following steps: putting copper (II) porphyrin, potassium carbonate, potassium iodide and a hybrid silicon carrier into N,N-dimethylformamide, stirring, and carrying out an immobilization reaction for 1.0-120 h in a nitrogen atmosphere at the temperature of 50-200 DEG C; then carrying out suction filtration and centrifugation on the reaction system to obtain a solid substance; and carrying out vacuum drying on the obtained solid substance at the temperature of 60-120 DEG C for 8-36 h to obtain the heterogeneous copper porphyrin catalyst. A method comprises the steps: dispersing heterogeneous copper porphyrin in cycloalkane, sealing the reaction system, heating to 100-130 DEG C while stirring, introducing oxygento 0.2-3.0 MPa, keeping the set temperature and oxygen pressure, stirring to react for 3-24 h, and carrying out after-treatment on the reaction solution to obtain products naphthenic alcohol and naphthenone. The naphthenic alcohol and the naphthenone are high in selectivity, generation of aliphatic diacid is effectively inhibited, and the method is a novel efficient and feasible cycloalkane selective catalytic oxidation method.
Owner:ZHEJIANG UNIV OF TECH
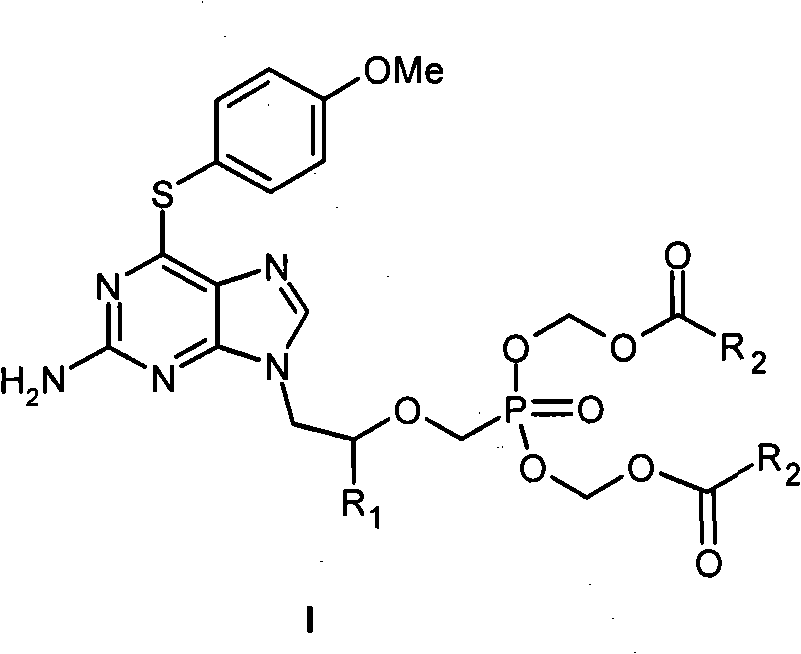
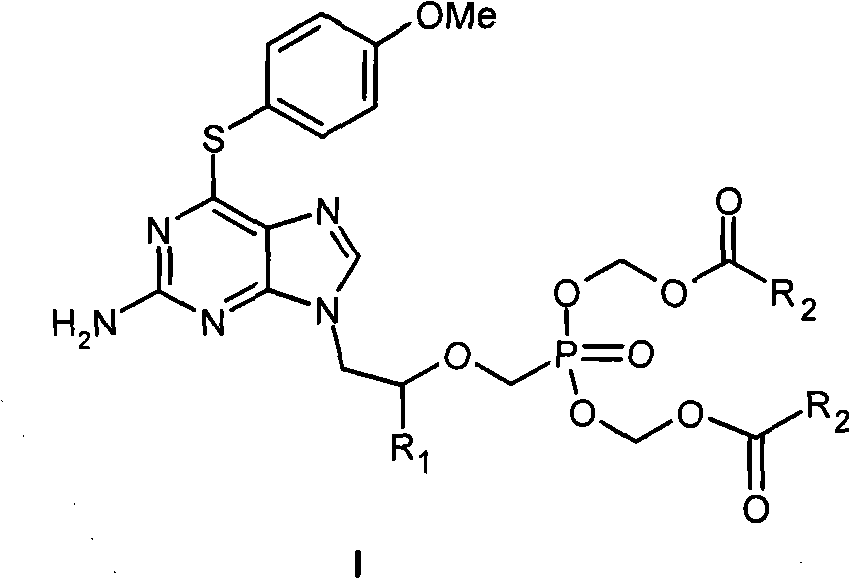
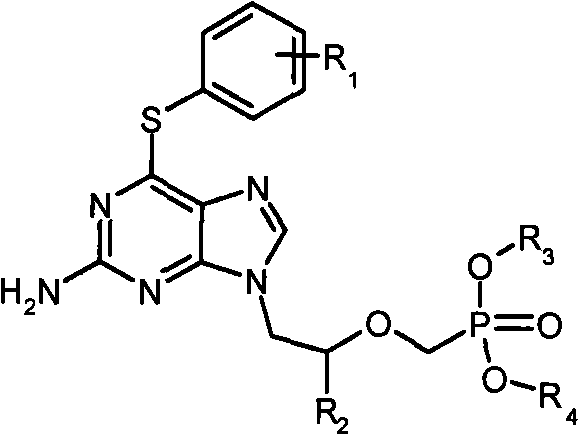
Acyclic nucleoside phosphonate derivative and medicinal application thereof
ActiveCN102093422AOrganic active ingredientsGroup 5/15 element organic compoundsCytotoxicityPharmaceutical medicine
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Non-aromatic based antioxidants for lubricants
InactiveUS20130288939A1Minimize changesThermoxidative stabilityOrganic chemistryAdditivesPolymer sciencePolyolefin
A lubricant composition comprises a cycloaliphatic amine alkoxylate and a base oil selected from (a) a polyalkylene glycol; (b) a polyalphaolefin; (c) a naphthenic compound; and (d) combinations thereof; provided that the cycloaliphatic amine alkoxylate and the base oil are miscible. The lubricant may exhibit a viscosity change at 40° C. of less than 5 percent (%) after 13 days at 120° C. in dry air, indicating significant thermoxidative stability with minimal or no aromatic content, thereby enabling use of Groups II and III base oils, in particular, without reduced toxicity and / or quality concerns as compared with Group I base oil lubricants.
Owner:DOW GLOBAL TECH LLC
Process for producing cycloalkanol and/or cycloalkanone
InactiveUS20060020149A1Good choicePreparation by oxidation reactionsOrganic compound preparationHalohydrocarbonAcyl group
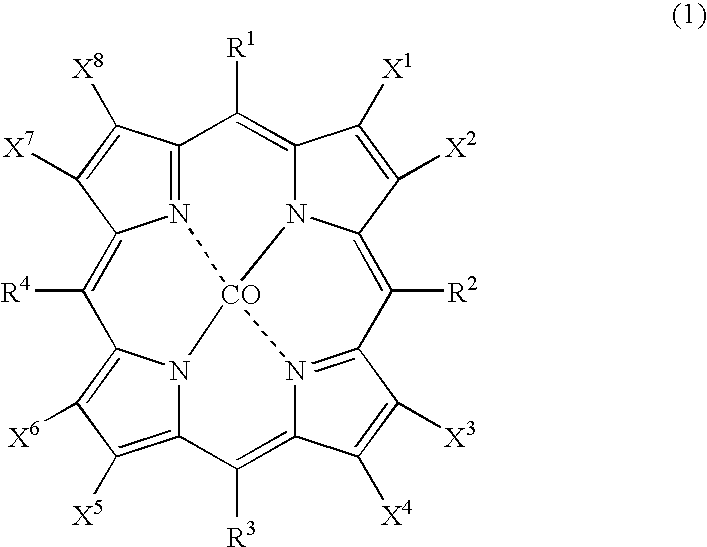
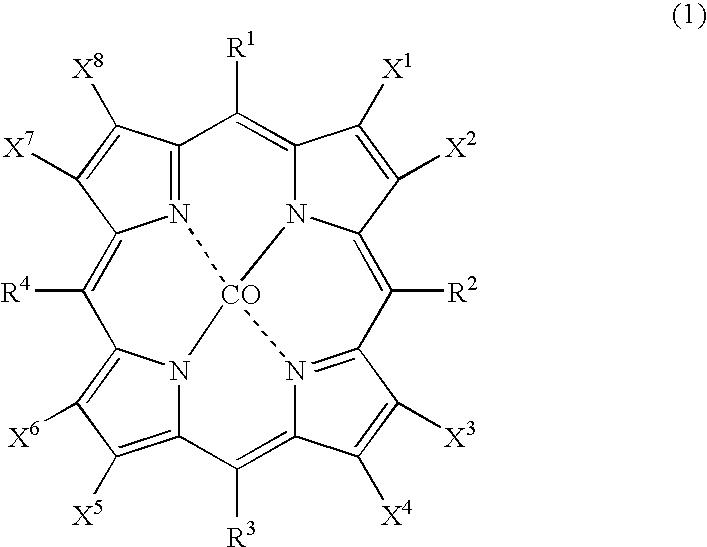
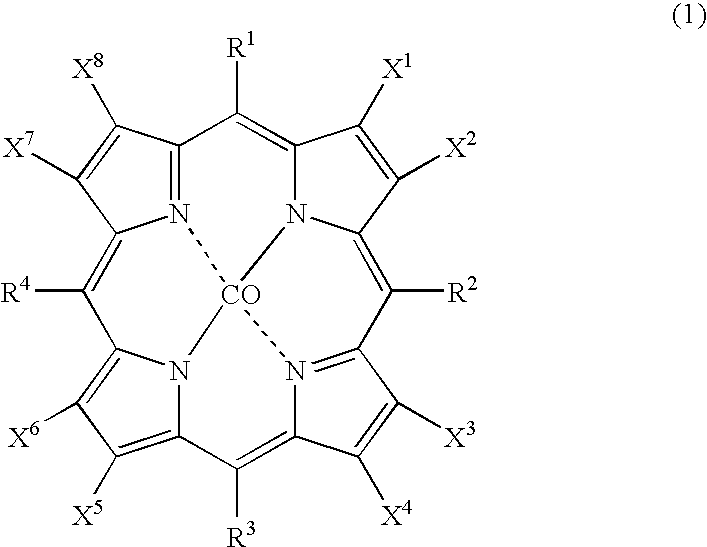
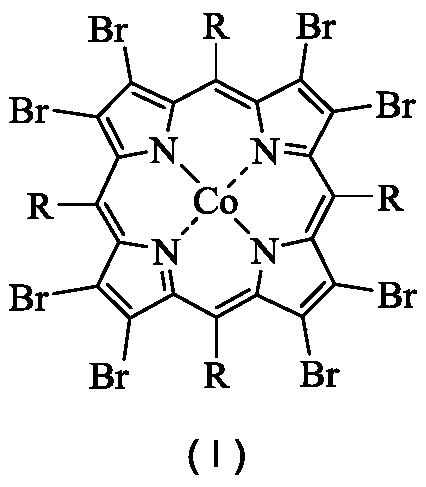
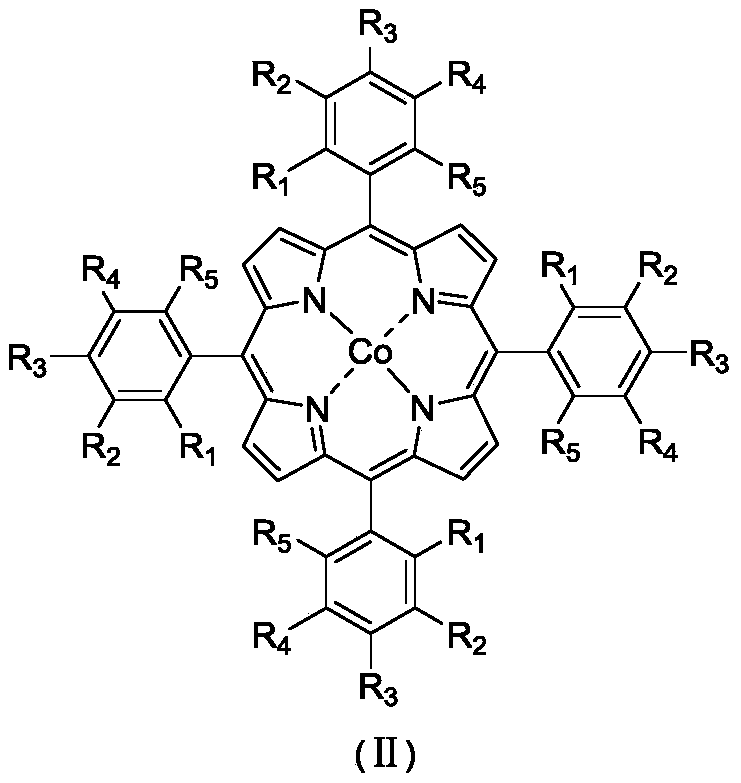
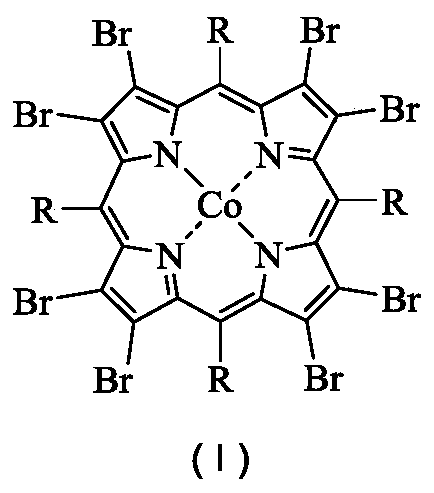
The object of the present invention is to produce cycloalkanol and / or cycloalkanone with good selectivity by oxidizing cycloalkane with molecular oxygen. The method of the present invention comprises the step of oxidizing cycloalkane with molecular oxygen in the presence of cobalt salt of carboxylic acid and cobalt complex with porphyrin as a ligand; such cobalt complex typically represented by the formula (1): (wherein, each of X1 to X8 independently represents a hydrogen atom, a halogen atom, a nitro group, a cyano group, a hydrocarbon group, a halogenated hydrocarbon group or a sulfonyl group, each of R1 to R4 independently represents a hydrogen atom, a nitro group, a cyano group, a hydrocarbon group or a halogenated hydrocarbon group.)
Owner:SUMITOMO CHEM CO LTD
Method for catalytic oxidation of cycloalkane by confinement porphyrin Co (II)
PendingCN111018673AImprove conversion rateImprove stabilityPreparation by oxidation reactionsOrganic compound preparationAlcoholPorphyrin
The invention relates to a method for catalytic oxidation of cycloalkane by confinement porphyrin Co (II). The method comprises the following steps: dispersing confinement cobalt porphyrin (II) in cycloalkane, sealing the reaction system, heating to 100-130 DEG C while stirring, introducing oxygen to 0.2-3.0 MPa, keeping the set temperature and oxygen pressure, stirring to react for 3.0-24.0 h, and carrying out post-treatment on a reaction solution to obtain products naphthenic alcohol and naphthenic ketone. The method achieves high selectivity of naphthenic alcohol and naphthenic ketone, andeffectively inhibits the generation of aliphatic diacid. The aliphatic diacid is low in selectivity, so that the continuity of the cycloalkane oxidation process and the separation of the products arefacilitated; the method has the potential of solving the problem that naphthenic alcohol and naphthenic ketone are easily and deeply oxidized to generate aliphatic diacid in the industrial cycloalkanecatalytic oxidation process; and the method is a novel efficient and feasible method for selective catalytic oxidation of cycloalkane.
Owner:ZHEJIANG UNIV OF TECH
Process for producing a 2-alkyl-2-cycloalken-1-one
ActiveUS20110040127A1Organic compound preparationCatalyst activation/preparationPtru catalystProcess engineering
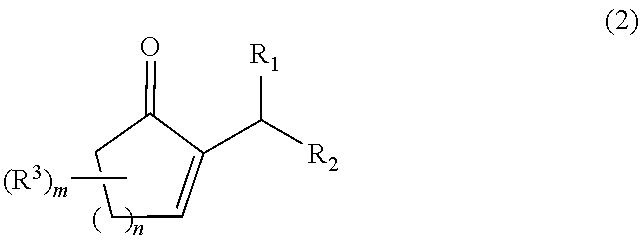
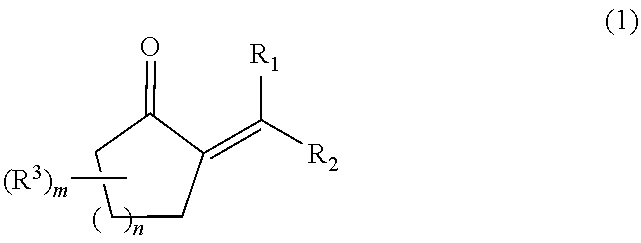
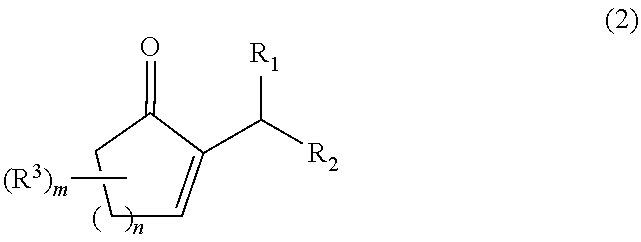
The present invention relates to a process for producing a 2-alkyl-2-cycloalken-1-one represented by the following general formula (2), including the step of reacting a 2-alkylidene cycloalkanone in the presence of a palladium and / or platinum catalyst which is treated in the following steps (a) and (b); and a method for activating the palladium and / or platinum catalyst including the following steps (a) and (b):Step (a): activating the palladium and / or platinum catalyst in an atmosphere containing a hydrogen gas; andStep (b): replacing the hydrogen gas being present as the atmosphere for the catalyst in the step (a), with an inert gas to remove the hydrogen gas out of the reaction system,wherein m is 0 to 3; n is 1 or 2; R1 and R2 are each independently a hydrogen atom or an alkyl group having 1 to 8 carbon atoms; and R3 is an alkyl group having 1 to 5 carbon atoms. In accordance with the present invention, the 2-alkyl-2-cycloalken-1-one can be produced with a high purity and a high productivity.
Owner:KAO CORP
Peroxisome proliferator activated receptor agonists
InactiveUS7544812B2Less side effectsLowering fibrinogenOrganic active ingredientsBiocideDouble bondPeroxisome proliferator-activated receptor
The present invention is directed to compounds represented by the following structural Formula (I), (a) R1 is selected from the group consisting of hydrogen, substituted or unsubstituted group selected from C1-C8 alkyl, aryl-C0-4-alkyl, heteroaryl-C0-4-alkyl, C3-C6 cycloalkylaryl-C0-2-alkyl, and —CH2—C(O)—R17-R18, wherein R17 is O or NH and R18 is optionally substituted benzyl; (b) R2 is selected from the group consisting of C1-C6 alkyl, C1-C6 alkenyl, aryl-C0-4-alkyl, heteroaryl-C0-4-alkyl, C1-C4 alkyl sulfonamide, C1-C4 alkyl amide, OR10 and C3-C6 cycloalkyl; (c) W is O or S; (d) X is an optionally substituted C1-C5 alkylene linker wherein one carbon atom of the linker may optionally be replaced with O, NH, S, and optionally two carbons together may form a double bond; (e) Y is selected from the group consisting of C, O, S, NH and a single bond; and (f) E is selected from the group consisting of C(R3)(R4)A, A, and a substituted or unsubstituted group selected from the group consisting of (CH2)n COOR19.
Owner:ELI LILLY & CO
Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors
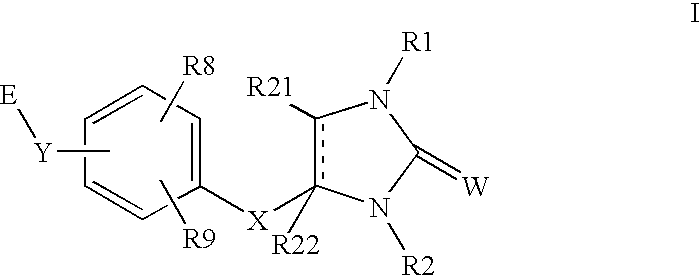
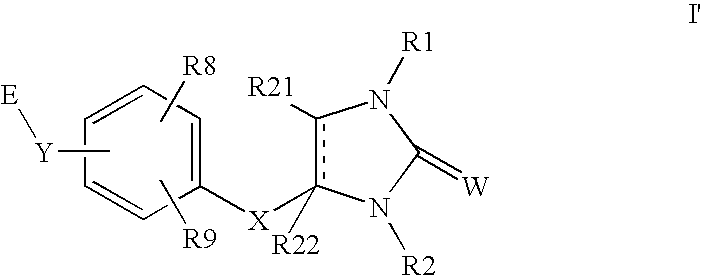
InactiveUS7846935B2Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryDiseaseGingival disease
Owner:AMURA THERAPEUTICS
TRPV3 small molecule allosteric inhibitor and preparation method thereof
ActiveCN112480018AAdd structure typesOrganic active ingredientsOrganic chemistryMorpholineAcyl group
The present invention discloses a TRPV3 small molecule allosteric inhibitor and a preparation method thereof. The small molecule inhibitor comprises compounds represented by a general formula (I) anda general formula (II) shown in the specification or pharmaceutically acceptable salts thereof, wherein X is selected from N or C, Y is selected from (CH2) or (CO); R2 is selected from hydrogen and halogen; R4 is selected from C1-C8 alkyl groups, C3-C8 cycloalkyl groups, a naphthalene ring, an adamantyl group, morpholine, 4-(methylsulfonyl) piperazine and 4-(trifluoromethyl)pyridine-2-amine; and R1 and R3 are respectively and independently selected from hydrogen, deuterium, halogen, hydroxyl, sulfydryl, cyano, nitro, C1-C8 alkyl, halogenated C1-C8 alkyl, C1-C8 alkoxy, C3-C8 naphthenic bases, C3-C8 cycloalkenyl, C2-C8 alkenyl, C2-C8 alkynyl, C6-C10 aryl, C3-C10 heteroaryl and C4-C8 heterocyclic radical.
Owner:CHINA PHARM UNIV
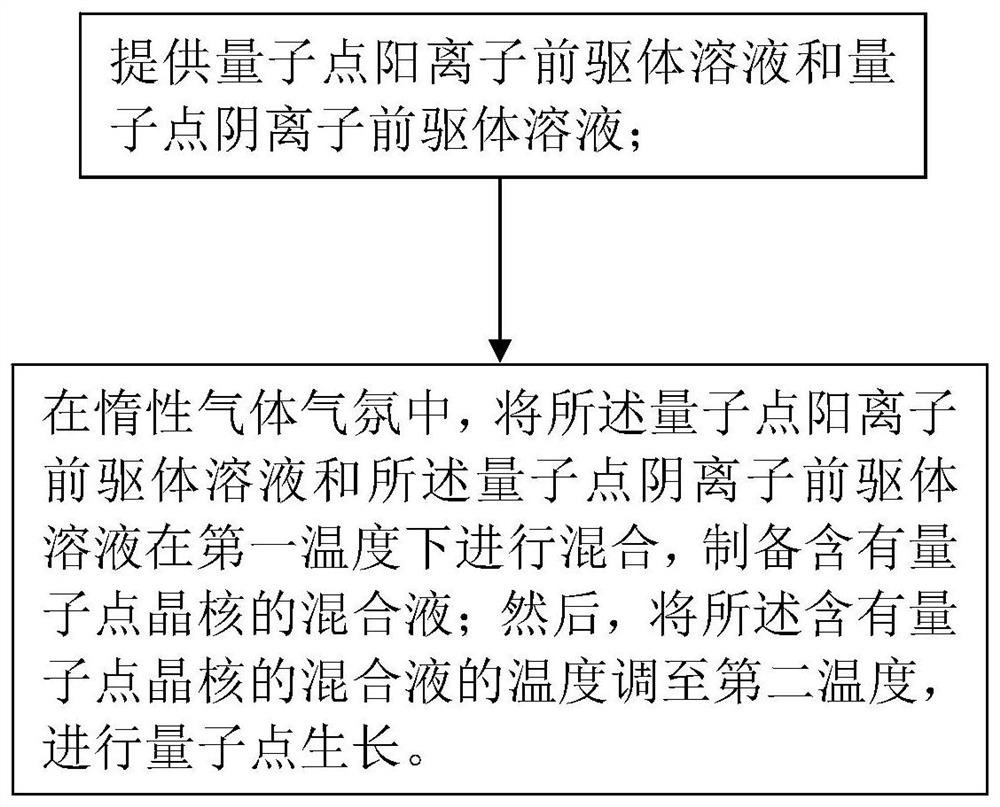
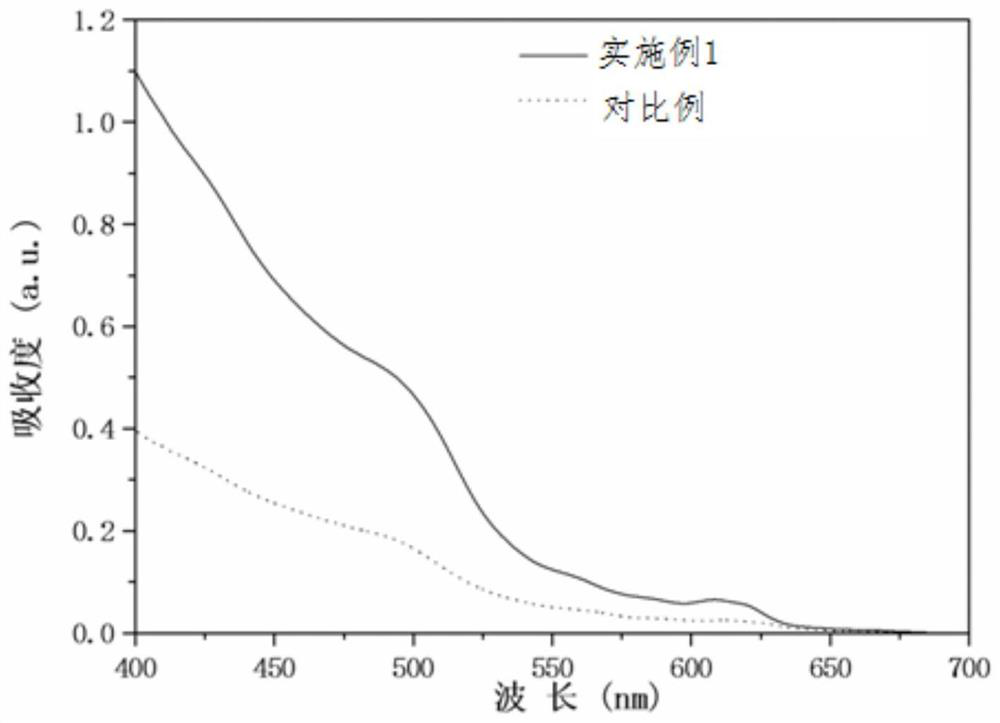
Preparation method of quantum dot material
InactiveCN112538353AHigh reactivityImprove conversion rateNanoopticsSelenium/tellurium compounds with other elementsChemical physicsPhysical chemistry
The invention belongs to the technical field of panel display, and particularly relates to a preparation method of a quantum dot material. The preparation method provided by the invention comprises the following steps of: providing a quantum dot cationic precursor solution and a quantum dot anionic precursor solution; in an inert gas atmosphere, mixing the quantum dot cation precursor solution andthe quantum dot anion precursor solution at a first temperature to prepare a mixed solution containing quantum dot crystal nucleuses; and adjusting the temperature of the mixed solution containing the quantum dot crystal nucleus to a second temperature, and carrying out quantum dot growth. In the step of mixing the quantum dot cationic precursor solution and the quantum dot anionic precursor solution at the first temperature, a mixture of primary amine and secondary phosphine is added to be mixed with the quantum dot cationic precursor solution and / or the quantum dot anionic precursor solution, wherein the secondary phosphine has a general formula RR'PH2, R is selected from alkyl or cycloalkyl, and R' is selected from alkyl or cycloalkyl. According to the method, a mixture of primary amine and secondary phosphine is added, so that high-yield synthesis of the quantum dot material is realized.
Owner:TCL CORPORATION
Alpha-amino cyclo nitrile compound preparation method
InactiveCN102731236ALow costShorten the production cycleCarboxylic acid nitrile preparationOrganic compound preparationCyanide compoundPtru catalyst
The invention discloses an alpha-amino cyclo nitrile compound preparation method. Cycloalkanone, organic amine salt or inorganic amine salt, and alkali metal cyanide or acetone cyanohydrin undergo a reaction with stirring in water at the temperature of 20 DEG C to 30 DEG C in the absence of a catalyst to prepare the alpha-amino cyclo nitrile compound. According to the method, the cycloalkanone, the organic amine salt or the inorganic amine salt, and the alkali metal cyanide or the acetone cyanohydrin are used as reaction raw materials which are cheap and easy to obtain; the water is used as a reaction solvent which is environment-friendly and has low cost; no catalyst is used, which has low cost and has no subsequent catalyst recovery trouble; the alpha-amino cyclo nitrile compound is prepared by a one-pot method, and the preparation route is short and the operation is simple; a reaction liquid only needs to undergo simple steps such as organic solvent extroperation, washing, dewatering and drying, organic solvent recovery and the like to obtain the high qualified product, and complicated processing steps such as column chromatography purification is not necessary; the prepared product has high purity of more than 91% and high yield of more than 90%; and the method can be operated continuously, has great production capacity, short production cycle and short production cost, and can be applied to industrialized mass production.
Owner:CHONGQING UNISPLENDOUR CHEM
Method for manufacturing cycloalkanol and/or cycloalkanone
InactiveUS7166751B2Good coefficientFavorable degree of conversionPreparation by oxidation reactionsOrganic compound preparationPtru catalystCerium(IV) oxide
The object of the present invention is to provide a method for manufacturing cycloalkanol and / or cycloalkanone with favorable selectivity coefficient by oxidizing cycloalkane with favorable degree of conversion.In the present invention, cycloalkane is oxidized with oxygen in the presence of a catalyst such that gold is supported on ceric oxide. Said oxidation is preferably performed in the presence of a free-radical initiator and the free-radical initiator is preferably 2,2′-azobis(isobutyronitrile).
Owner:SUMITOMO CHEM CO LTD
Method for preparing substituted alkyl cycloalkanones
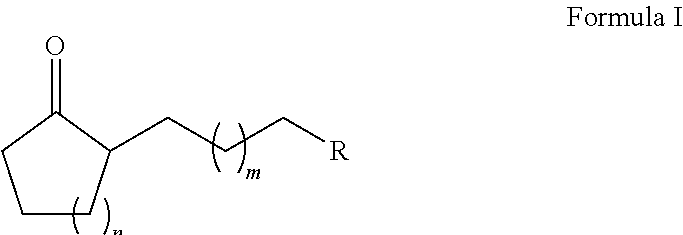
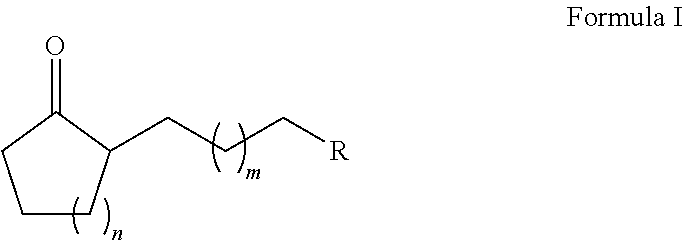
ActiveUS20180022683A1Reduced metal oxide can be reoxidized particularly effectivelyRapid reoxidationOrganic compound preparationGroup 5/15 element organic compoundsAlkyl transferAlkyl cycloalkane
The present invention relates to a method for producing a substituted alkyl cycloalkanone of formula (I), having the alkylation of a cycloalkanone of formula (II) with an alkene derivative of formula (III) in the presence of a metal oxide, where n is 2 to 20, m is 0 to 10, and R is a functional group.
Owner:SYMRISE GMBH & CO KG
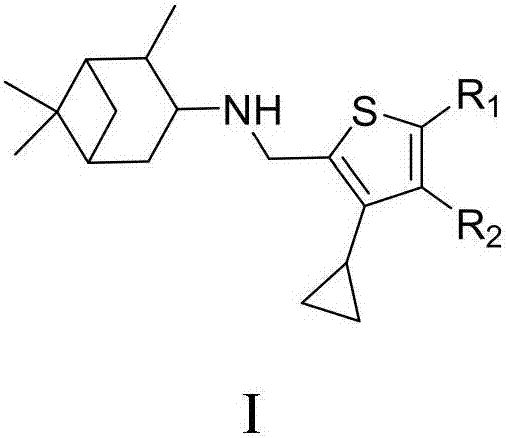
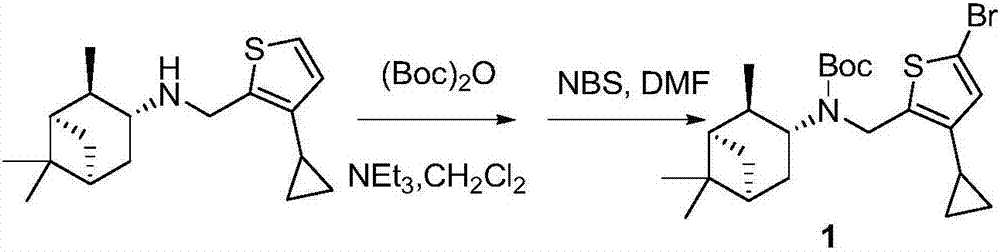
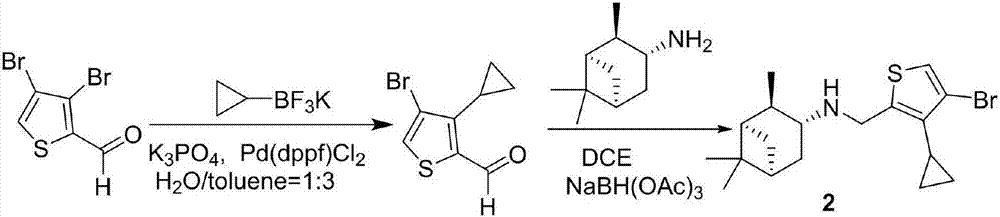
Cyclopropyl substituted thiophene naphthene amine type compound and application thereof
The invention discloses a cyclopropyl substituted thiophene naphthene amine type compound with a structure I as shown in the description, or pharmaceutically acceptable salts, stereisomers or prodrug molecules of the compound. The novel compound has a relatively good anti-influenza virus function, and has a relatively good inhibition function on influenza viruses with drug resistance to amantadine. The novel compound can be used for preventing and treating infection of influenza viruses.
Owner:GUANGZHOU MEDICAL UNIV +2
Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors
InactiveUS7803803B2Improve efficacyDesirable pharmacokinetic propertyAntibacterial agentsBiocideDiseaseCathepsin K
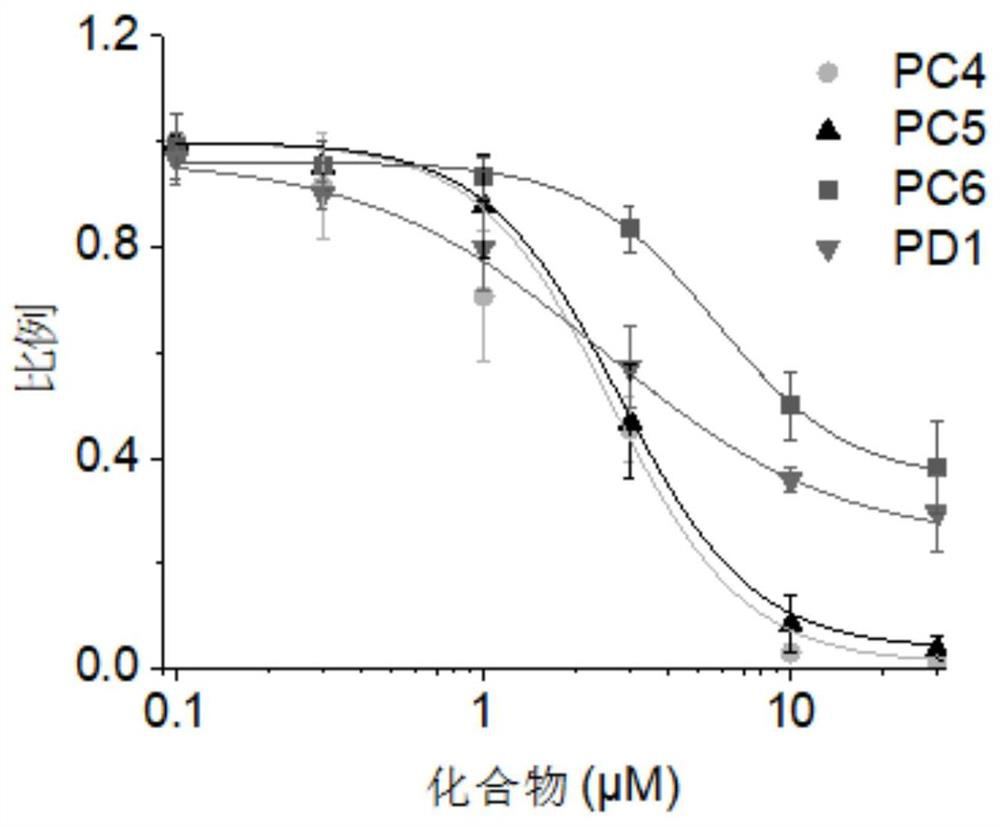
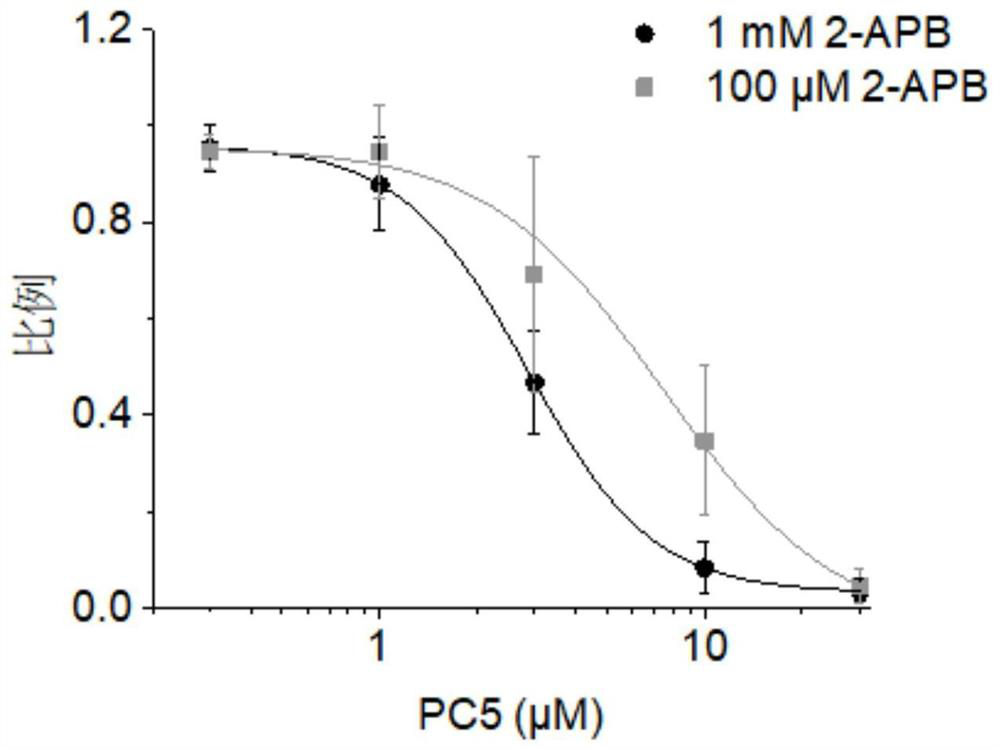
The present invention relates to compounds of formula (I), and pharmaceutically acceptable salts thereof,wherein:X is CH or N;one of R1 and R2 is H, and the other is selected from OR6, SR6, NR6R7, N3, Me, Et, CF3, SOR8 and SO2R8;R3 is selected from tert-butylmethyl, iso-propylmethyl, sec-butyl, tert-butyl, cyclopentyl and cyclohexyl;R4 is optionally substituted C1-8 alkyl or optionally substituted C3-8 cycloalkyl;R6 and R7 are each independently selected from H, C1-8-alkyl and C3-8-cycloalkyl, or R6 and R7 are linked to form a cyclic group together with the nitrogen to which they are attached; andR8 is C1-8-alkyl or C3-8-cycloalkyl.The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:AMURA THERAPEUTICS
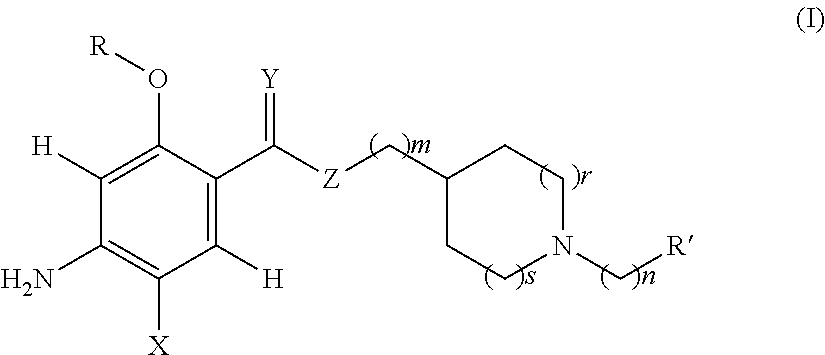
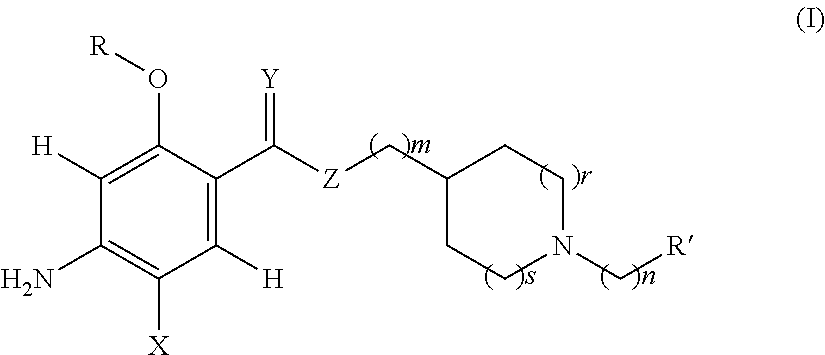
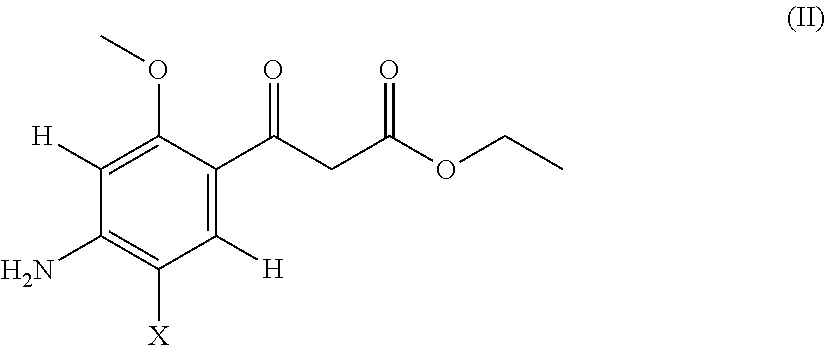
Acetylcholinesterase inhibitor compounds and 5ht4 serotonergic receptor agonists, with promnsia effect, methods for the preparation thereof and pharmaceutical compositions containing the same
Compounds are provided according to Formula (I)as well as their enantiomers and their racemics, their acid salts, their hydrates or their solvation products. Among a large number of possible meanings, X represents a halogen, Y an oxygen atom; all of the coefficients m, n, r and s have the value 1, R represents an ethyl and R′ a cycloalkyl. The invention also includes Methods of preparing the above compounds and the pharmaceutical compositions containing them also are provided.
Owner:UNIV CAEN
Film forming composition
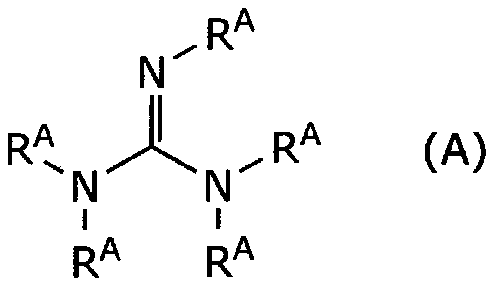
[Problem] To provide a film forming compositioncurable at low temperatureand a film forming method using the same. [Means for Solution] A film forming composition comprising a polysilazane, an organicsolvent and aspecificadditive, and a film formingmethod comprisingapplying itona substrate and curing. The specific additiveisselected from the group consisting of (A) guanidines substituted by a hydrocarbylgroup, (B) crown ether amines containing oxygen and nitrogen as a member thereof, (C) cycloalkanes having an amino-substituted polycyclic structure, (D) oximes substituted by a hydrocarbyl group, and (E) imidazolines.
Owner:MERCK PATENT GMBH
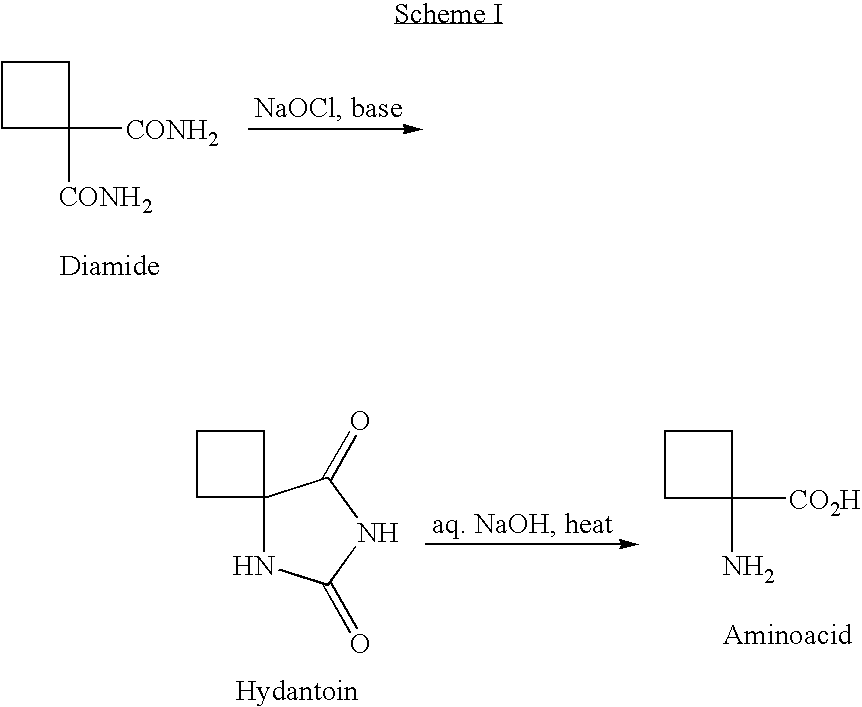
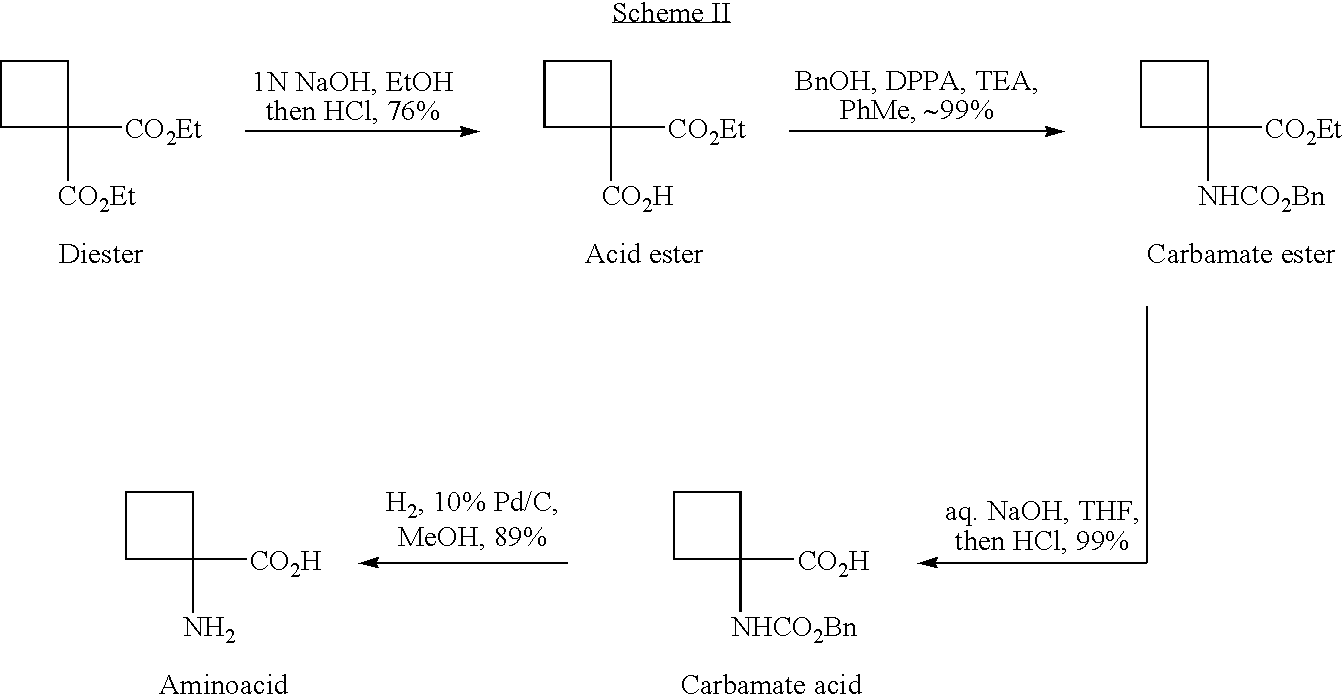
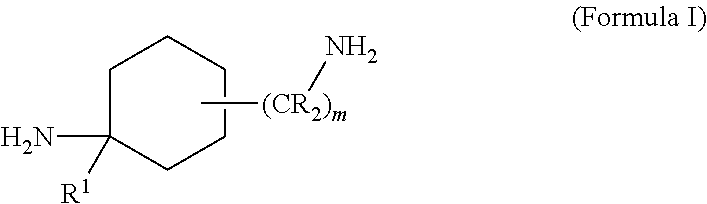
Cycloalkylamidoacid compounds, processes for making and uses thereof
The invention relates to the field of pharmaceutics and more specifically to novel cycloalkylamidoacid compositions useful in the preparation of cycloalkyaminoacids and oxazolidinediones, and processes for making cycloamidoacids. X and R are defined herein
Owner:BOEHRINGER INGELHEIM INT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
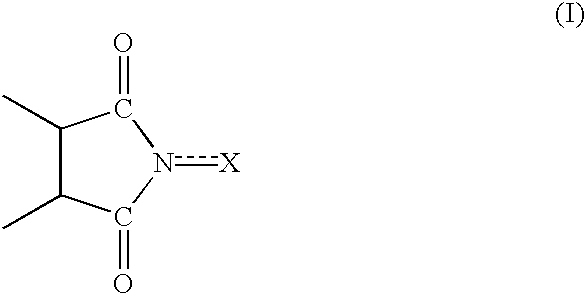
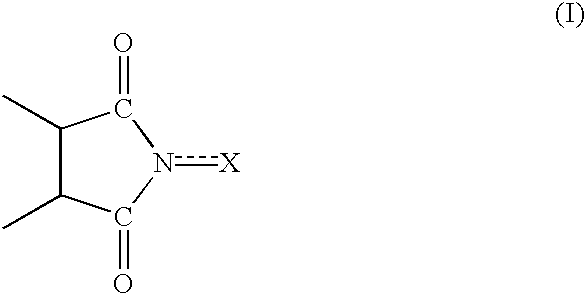
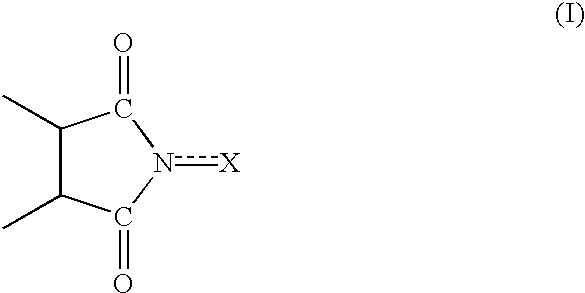



![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka.patsnap.com/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00001.png)
![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka.patsnap.com/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00002.png)
![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka.patsnap.com/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00003.png)
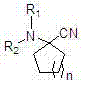
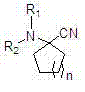

![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka.patsnap.com/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00001.png)
![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka.patsnap.com/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00002.png)
![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka.patsnap.com/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00003.png)