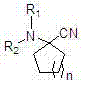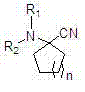Alpha-amino cyclo nitrile compound preparation method
A technology of aminocyclonitrile and compound, which is applied in the field of preparation of organic compounds, can solve problems such as increased organic solvent recovery cost, high price of organic cyanide, difficulty in catalyst recovery, etc., and achieves low cost, low production cost and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 30 grams of cyclohexanone, 0.306 mol of organic amine salt or 25 grams of ammonium bicarbonate into a 500 ml four-neck flask with airtight stirring, a thermometer and a dropping funnel, cool down to 0 ° C, and dropwise add Add 100 grams of sodium cyanide aqueous solution, dropwise in 40 minutes, slowly heat up to 20°C in 30 minutes, keep warm and stir for reaction, use gas chromatography to detect the disappearance of cyclohexanone as the end point of the reaction; after the reaction, extract the reaction solution with ether for 3 times , 150ml each time, combined the organic layers, washed 3 times with 50ml each time, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was rotary distilled to remove the solvent to obtain the product α-aminocyclohexane nitriles. The specific reaction conditions and results are shown in Table 1.
[0020] Table 1 Preparation of α-aminocyclohexane nitrile compounds
[0021]
Embodiment 2
[0023] Add 30 grams of cyclohexanone, 0.306 mol of organic amine salt or 25 grams of ammonium bicarbonate into a 500 ml four-neck flask with airtight stirring, a thermometer and a dropping funnel, cool down to 0 ° C, and dropwise add 133 grams of potassium cyanide aqueous solution was added dropwise in 40 minutes, slowly heated to 25°C in 30 minutes, kept warm and stirred for reaction, and the disappearance of cyclohexanone was detected by gas chromatography as the end point of the reaction; after the reaction, the reaction solution was extracted 3 times with ether , 150ml each time, combined the organic layers, washed 3 times with 50ml each time, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was rotary distilled to remove the solvent to obtain the product α-aminocyclohexane nitriles. The specific reaction conditions and results are shown in Table 2.
[0024] Table 2 Preparation of α-aminocyclohexane nitriles
[0025]
Embodiment 3
[0027] Add 30 grams of cyclohexanone, 0.306 mol of organic amine salt or 25 grams of ammonium bicarbonate, and 27 grams of acetone cyanohydrin into a 500 ml four-neck flask with airtight stirring, thermometer and dropping funnel, cool down to 0 °C, and add saturated carbonic acid dropwise. 220 grams of potassium aqueous solution was added dropwise in 40 minutes, slowly warming up to 30° C. in 30 minutes, insulated and stirred for reaction, and the disappearance of cyclohexanone was detected by gas chromatography as the end point of the reaction; after the reaction was finished, the reaction solution was extracted 3 times with ether, 150ml each time, combined the organic layers, washed 3 times with 50ml each time, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was rotary distilled to remove the solvent to obtain the product α-aminocyclohexane nitrile compound. The specific reaction conditions and results are shown in Table 3.
[0028] Table 3 Prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com