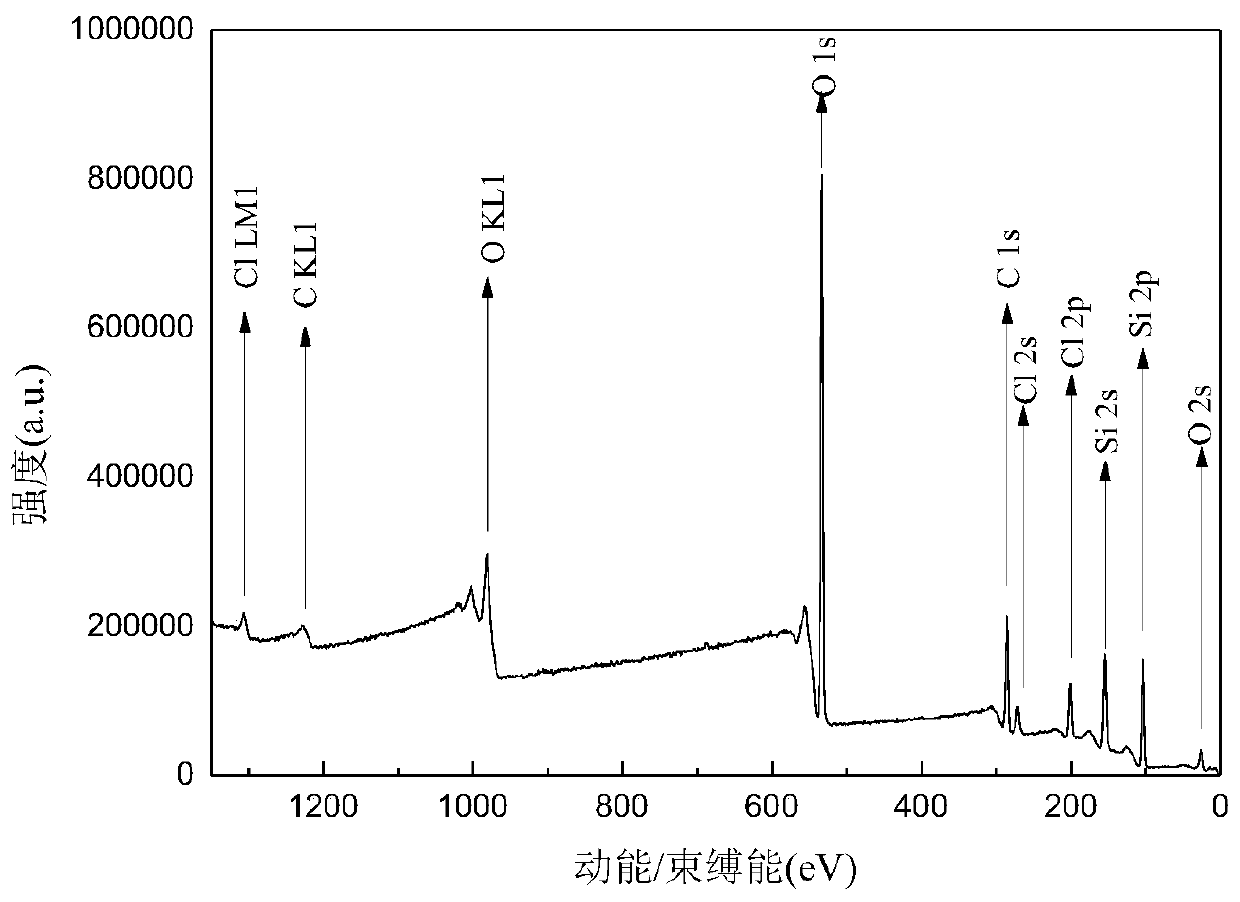
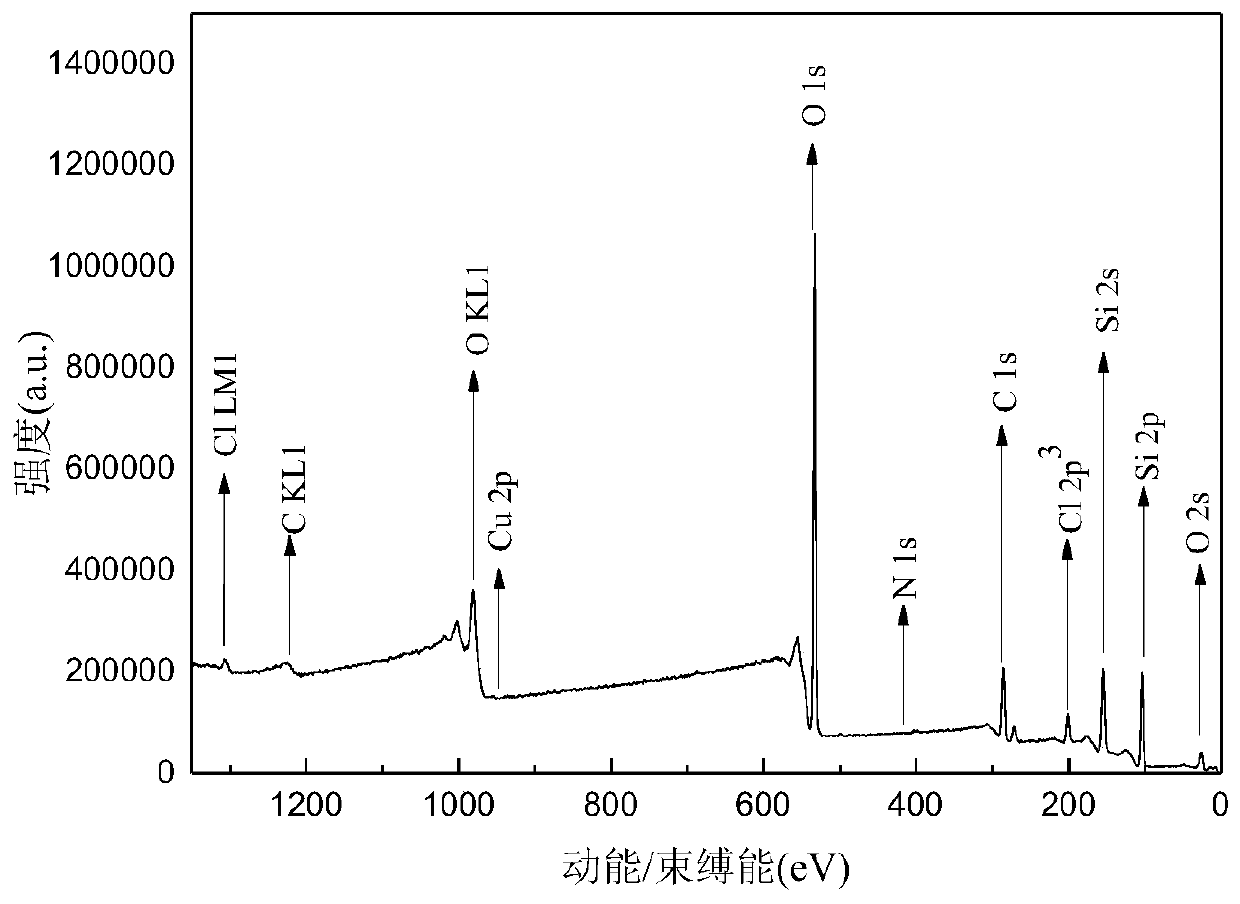
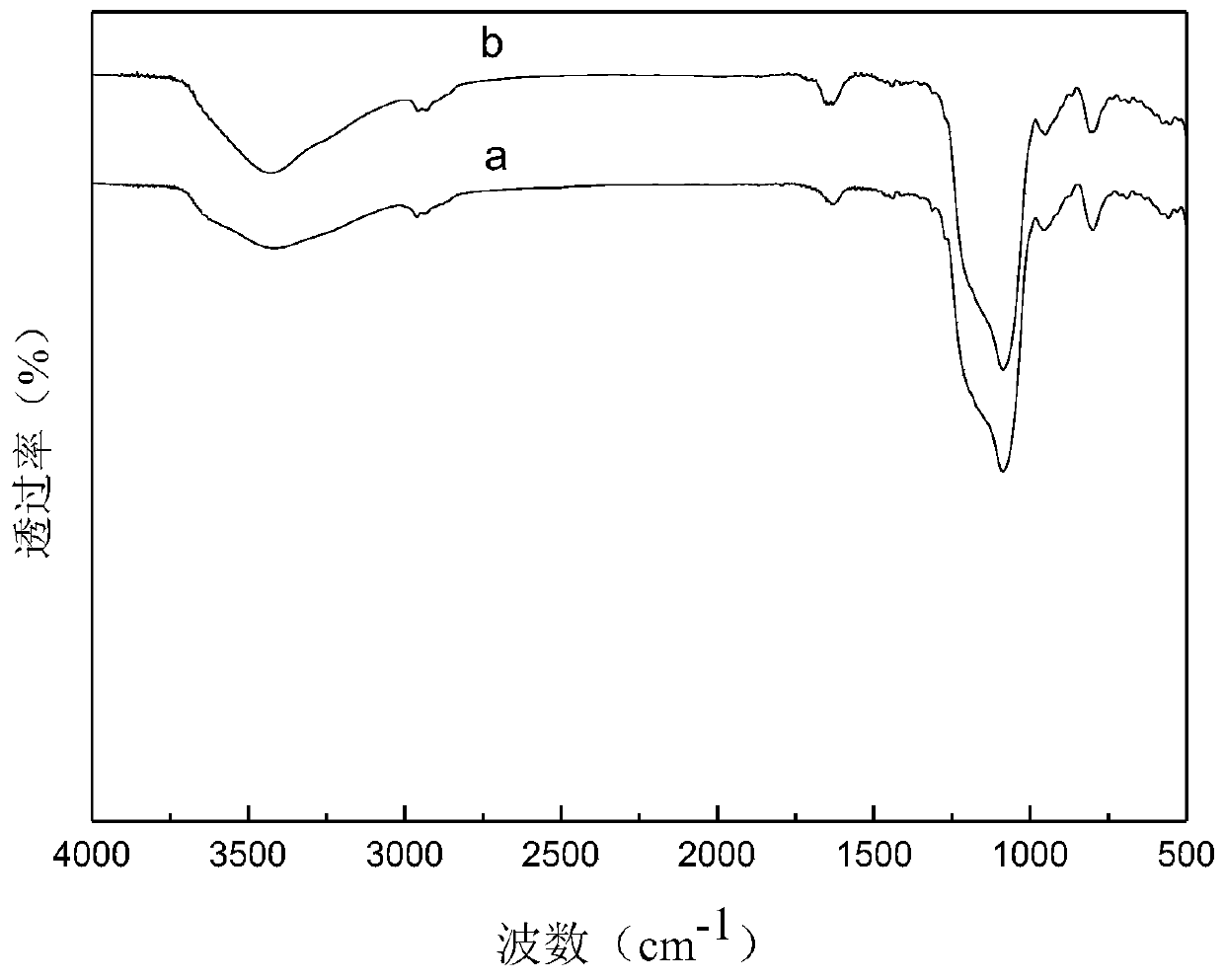
Heterogeneous copper porphyrin as well as preparation method and application thereof
A heterogeneous, porphyrin technology, applied in the direction of oxidation reaction preparation, peroxy compound preparation, carbon-based compound preparation, etc., to achieve the effect of inhibiting formation, novel structure and low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 49
[0040] Example 49 is a scale-up experiment of the application of the heterogeneous copper porphyrin catalyst in the catalytic oxidation reaction of naphthenes.
[0041] The preparation method of the hybrid silicon carrier of the present invention can be found in J.Mol.Catal.A-Chem.2010,319,58; Eur.J.Org.Chem.2012,3625; React.Funct.Polym.2013,73,192 , specifically: Dissolve 12.04g of 3-chloropropyltriethoxysilane and 41.67g of ethyl orthosilicate in 150mL of absolute ethanol to obtain solution ①; mix 17.12g of water and 20.0mL of 1M tetrabutyl fluoride The tetrahydrofuran solution of ammonium chloride was dissolved in 100mL of absolute ethanol to prepare solution ②; quickly pour solution ① into solution ②, shake vigorously for 10 seconds, let stand, and age at room temperature for 6.0 days. The obtained solid was washed with absolute ethanol (3×150 mL), and then vacuum-dried at 80° C. for 10 h to obtain 46.58 g of white solid powder (hybrid silicon carrier).
[0042] The metal...
Embodiment 1
[0044] In a 50mL single-port reaction tube, 2.0000g hybrid silicon support, 0.2400g (0.3mmol) 5,10,15-tris(p-chlorophenyl)-20-(p-hydroxyphenyl)porphyrin copper(II ), 0.1200g (0.9mmol) K 2 CO 3 and 0.0300g (0.2mmol) KI were placed in 20mL N,N-dimethylformamide, stirred at room temperature under nitrogen atmosphere for 10min, then the reaction mixture was stirred and heated to 50°C, and stirred for 72.0h. Cool to room temperature, filter with suction, transfer to a 10mL centrifuge tube, centrifuge for 10min, remove the solid in the lower layer, dry DMF and wash (10×8mL) until the upper layer is clear, wash with distilled water (2×8mL) until the upper layer is clear, dry and wash with acetone ( 5×8mL) until the upper layer was clear, and the solid in the lower layer was taken and dried at 80°C for 12.0h. 0.3567 g of pink solid powder (Si@p-Cl-Porp.) was obtained.
Embodiment 2
[0046] In a 50mL single-port reaction tube, 2.0000g hybrid silicon support, 0.2400g (0.3mmol) 5,10,15-tris(p-chlorophenyl)-20-(p-hydroxyphenyl)porphyrin copper(II ), 0.1200g (0.9mmol) K 2 CO 3 and 0.0300g (0.2mmol) KI were placed in 20mL N,N-dimethylformamide, stirred at room temperature under nitrogen atmosphere for 10min, then the reaction mixture was stirred and heated to 120°C, and stirred for 72.0h. Cool to room temperature, filter with suction, transfer to a 10mL centrifuge tube, centrifuge for 10min, remove the solid in the lower layer, dry DMF and wash (10×8mL) until the upper layer is clear, wash with distilled water (2×8mL) until the upper layer is clear, dry and wash with acetone ( 5×8mL) until the upper layer was clear, and the solid in the lower layer was taken and dried at 80°C for 12.0h. 0.5259 g of pink solid powder (Si@p-Cl-Porp.) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com