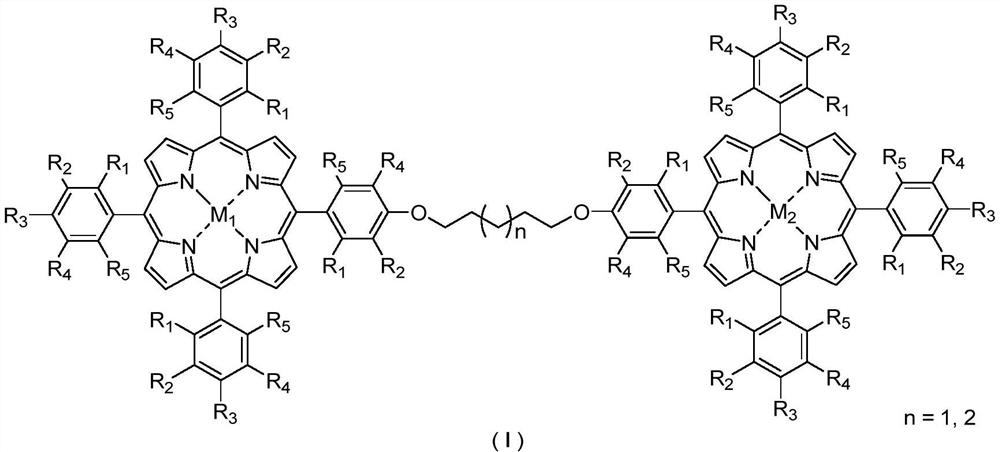
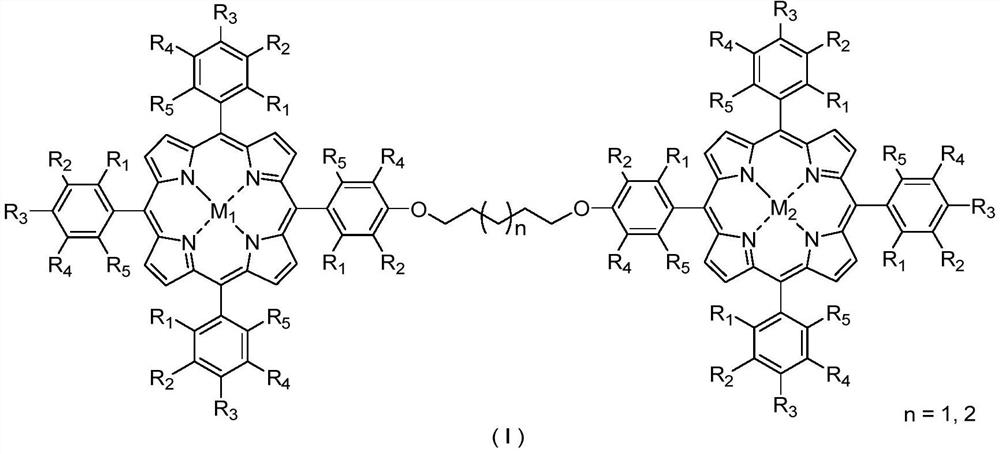
Bimetallic center metalloporphyrin as well as preparation method and application thereof
A metalloporphyrin and bimetallic technology, applied in the fields of organic catalysis and fine organic synthesis, can solve the problems of reducing the selectivity of cycloalkyl alcohols and cycloalkyl ketones, increasing the uncontrollability of the reaction system, and being unfavorable for continuous production, etc. Achieve high selectivity, is conducive to product separation, and is conducive to the effect of continuous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 66
[0032] Example 66 is a scale-up experiment of the application of the phase bimetallic center metalloporphyrin catalyst in the catalytic oxidation reaction of naphthenes.
[0033] The metalloporphyrins used in the present invention are synthesized with reference to Journal of the American Chemical Society 2017, 139: 18590-18597; Journal of the American Chemical Society 2018, 140: 6383-6390. All reagents used were commercially available of analytical grade.
Embodiment 1
[0035] In a 25mL reaction tube, 0.3189g (0.4mmol) 5,10,15-tris(p-chlorophenyl)-20-(p-hydroxyphenyl)porphyrin zinc (Ⅱ), 0.3156g (0.4mmol) 5,10,15-tris(p-chlorophenyl)-20-(p-hydroxyphenyl)porphyrin cobalt (Ⅱ), 0.0975g (0.4mmol) 1,6-dibromohexane, 0.0083g (0.04 mmol) KI, 0.1106g (0.8mmol) K 2 CO 3 , dissolved in 6mL DMF (except water), N 2 Under protection, react at 100°C for 72h. After the reaction was over, the solution was desolvated under reduced pressure. The resulting solid was dissolved in 300 mL of dichloromethane, washed with 4 × 300 mL of distilled water, anhydrous Na 2 SO 4 After drying, it was desolvated under reduced pressure. Silica gel column chromatography (200-300 mesh, packed with cyclohexane), V 环己烷 :V 二氯甲烷 =9:1, identify the eluent received by thin-layer chromatography (visible light color development), stop receiving when the color of the product becomes light, and collect a single point of the component, decompression precipitation, vacuum After dry...
Embodiment 2
[0037] In a 25mL reaction tube, 1.5941g (2mmol) 5,10,15-tris(p-chlorophenyl)-20-(p-hydroxyphenyl)porphyrin zinc (Ⅱ), 0.3156g (0.4mmol) 5 ,10,15-tris(p-chlorophenyl)-20-(p-hydroxyphenyl)porphyrin cobalt (Ⅱ), 0.0975g (0.4mmol) 1,6-dibromohexane, 0.0083g (0.04mmol )KI, 0.1106g (0.8mmol)K 2 CO 3 , dissolved in 6mL DMF (except water), N 2 Under protection, react at 100°C for 72h. After the reaction was over, the solution was desolvated under reduced pressure. The resulting solid was dissolved in 300 mL of dichloromethane, washed with 4 × 300 mL of distilled water, anhydrous Na 2 SO 4 After drying, it was desolvated under reduced pressure. Silica gel column chromatography (200-300 mesh, packed with cyclohexane), V 环己烷 :V 二氯甲烷 =9:1, identify the eluent received by thin-layer chromatography (visible light color development), stop receiving when the color of the product becomes light, and collect a single point of the component, decompression precipitation, vacuum After drying...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com