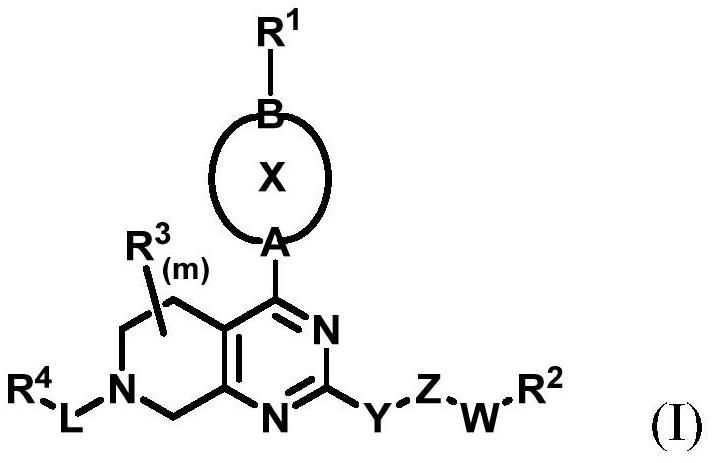
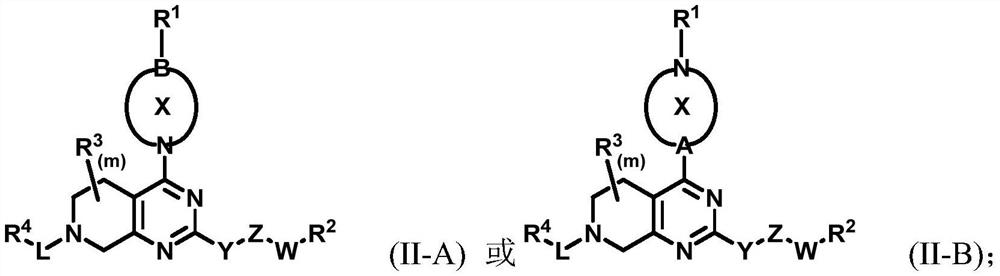
Cycloalkyl and heterocycloalkyl inhibitors as well as preparation method and application thereof
A technology of heterocycloalkyl and cycloalkyl is applied in the field of cycloalkyl and heterocycloalkyl inhibitors and their preparation, and can solve the problems of no drug approval and marketing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0221]The present invention also provides a preparation method of a pharmaceutical composition, comprising the steps of: mixing a pharmaceutically acceptable carrier with the compound of general formula (I) or its crystal form, pharmaceutically acceptable salt, hydrate or The solvates are mixed to form a pharmaceutical composition.
[0222] The present invention also provides a treatment method, which includes the steps of: administering the compound of general formula (I) described in the present invention, or its crystal form, pharmaceutically acceptable salt, hydrate or solvate to the subject in need of treatment , or administer the pharmaceutical composition of the present invention for selectively inhibiting KRAS G12C .
[0223] Compared with the prior art, the present invention has the following main advantages:
[0224] (1) The compound is to KRAS G12C Has a good selective inhibitory effect;
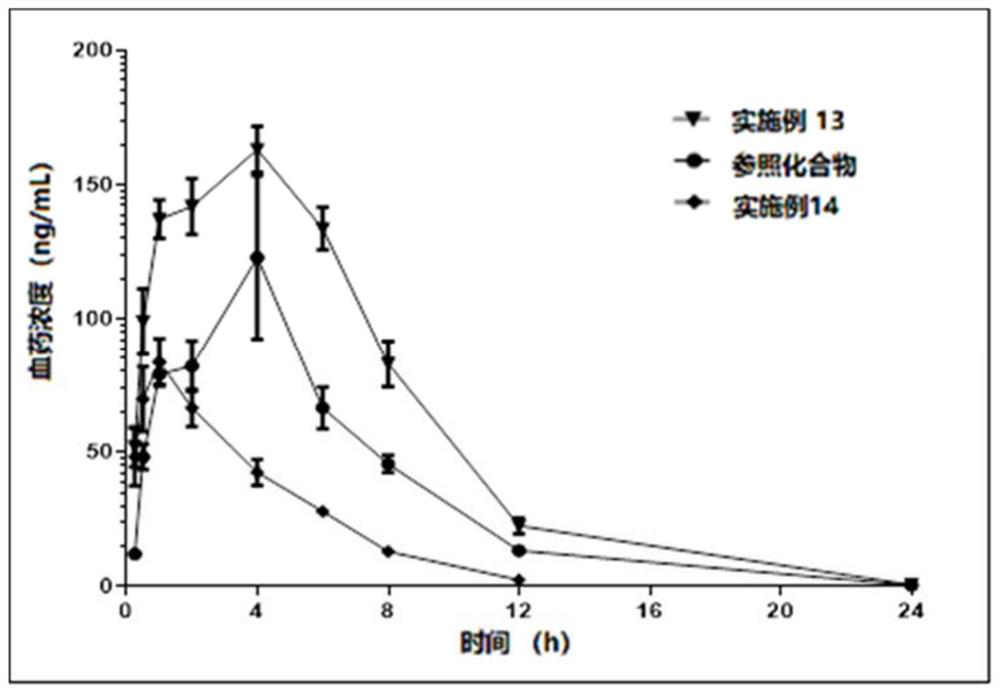
[0225] (2) The compound has better pharmacodynamics, pharmacokinetic prop...
Embodiment 1
[0386] Example 1 2-(1-acryloyl-4-(2-((R)-2-(cyclobutyl(methyl)amino)-3-methoxypropoxy)-7-(8-methyl Preparation of (1-naphthalen-1-yl)-5,6,7,8-tetrahydropyridin[3,4-d]pyrimidin-4-yl)piperazin-2-yl)acetonitrile
[0387]
[0388] The first step: 2-(cyanomethyl)-4-(2-((R)-2-(cyclobutyl(methyl)amino)-3-methoxypropoxy)-7-(8 Preparation of tert-butyl -methylnaphthalen-1-yl)-5,6,7,8-tetrahydropyridin[3,4-d]pyrimidin-4-yl)piperazine-1-carboxylate
[0389] 2-(cyanomethyl)-4-(7-(8-methylnaphthalen-1-yl)-2-(methylsulfoxide)-5,6,7,8-tetrahydropyridin[3 ,4-d]pyrimidin-4-yl)piperazine-1-carboxylic acid tert-butyl ester (72mg, 0.128mmol) was added to the reaction flask, followed by the addition of toluene (0.8mL), (S)-2-(cyclobutyl (methyl)amino)-3-methoxypropyl-1-ol (45mg, 0.256mmol) and sodium tert-butoxide (37mg, 0.384mmol). The reaction solution was stirred under ice-water bath for 0.5 h, then water (50 mL) was added, and then extracted with ethyl acetate (3 x 30 mL). All organic p...
Embodiment 2
[0398] Example 2 2-(1-acryloyl-4-(7-(8-chloronaphthalen-1-yl)-2-((1-((dimethylamino)methyl)cyclopropyl)methoxy) -5,6,7,8-tetrahydropyridin[3,4-d]pyrimidin-4-yl)piperazin-2-yl)acetonitrile
[0399]
[0400] LCMS:m / z 600(M+H) + . 1 HNMR (400MHz, CDCl 3 )δ7.76(m,1H),7.62(m,1H),7.52(m,1H),7.44(m,1H),7.33(m,1H),7.23(m,1H),6.58(m,1H ),6.41(d,J=16.8Hz,1H),5.84(d,J=10.4Hz,1H),5.08(brs,0.5H),4.61(brs,0.5H),4.41(m,1H),4.05 (m,6H),3.56(m,1H),3.42(m,1H),3.14(m,4H),2.40(m,11H),0.72(m,2H),0.54(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com