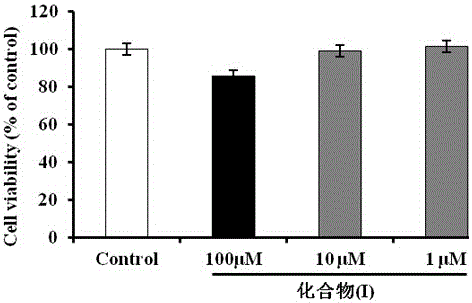
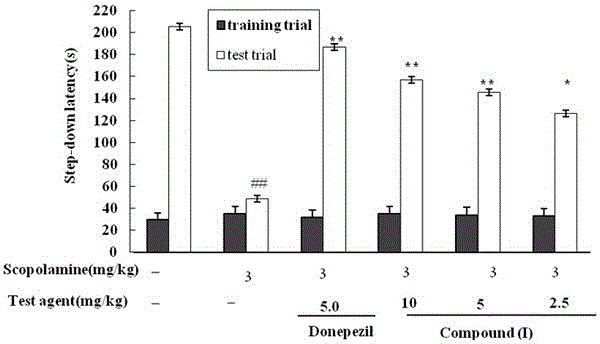
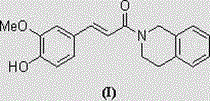
(N-1,2,3,4-tetrahydroisoquinolinyl)-feruloylagmatine compound, and preparation method and application thereof
A technology of tetrahydroisoquinolinyl and ferulamide, which is applied in the field of medicine, can solve problems such as dementia, neuron loss, and deterioration, and achieve good antioxidant activity, low toxicity, and the effect of inhibiting aggregation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0034] The preparation method of (N-1,2,3,4-tetrahydroisoquinolinyl)-ferulamide compound comprises the following steps:
[0035] ,
[0036] In the formula, Me represents a methyl group,
[0037] Add ferulic acid (1), condensing agent and solvent into the reaction flask, stir evenly, then add 1,2,3,4-tetrahydroisoquinoline (2), after the addition, stir and react at temperature T for n hours, TLC Monitoring; after the reaction, evaporate the solvent under reduced pressure, add water to the residue, extract with dichloromethane, wash the organic layers with saturated aqueous sodium chloride solution, dry over anhydrous sodium sulfate, filter, and evaporate the filtrate to remove the solvent under reduced pressure , and the residue was purified by silica gel column chromatography (eluent: petroleum ether / acetone=20 / 1) to obtain the target product (N-1,2,3,4-tetrahydroisoquinolinyl)-ferulamide ( I).
[0038](N-1,2,3,4-tetrahydroisoquinolinyl)-ferulamide (I) is a white solid wi...
Embodiment 1
[0044] (N-1,2,3,4-tetrahydroisoquinolinyl)-ferulamide (I) obtained in Example 1 of the present invention 1 H NMR, 13 C NMR and ESI-MS detection results are:
[0045] 1 H NMR (400 MHz, CDCl 3 ) δ 7.66 (d, J = 15.2 Hz, 1H, C=CH), 7.24-7.12 (m, 5H, 5× Ar-H), 7.16 (d, J = 1.6 Hz, 1H, Ar-H), 6.93 (d, J = 8.0 Hz, 1H, Ar-H), 6.80 (d, J =15.2 Hz, 1H, C=CH), 6.04 (s, 1H, OH), 4.84 (s, 2H, phCH 2 N), 3.94 (s, 3H, OCH 3 ), 3.88 (s, 2H, phCH 2 ), 3.97-2.91 (m, 2H, NCH 2 ), 3.80-3.74 (m, 4H, 2 × NCH 2 ).
[0046] 13 C NMR (100 MHz, CDCl 3 ) 166.24, 147.42, 146.74, 143.06, 134.25, 133.70, 128.97, 128.26, 127.81, 126.76, 126.14, 121.90, 114.79 (2C), 110.02, 56.02, 43.60, 29.74.
[0047] MS (ESI) m / z: 310.1 [M + H] + .
[0048] The present invention also provides a pharmaceutical composition for treating neurodegenerative diseases, comprising an effective amount of the above-mentioned drug for treating neurodegenerative diseases or a pharmaceutically acceptable hydrate the...
Embodiment 11
[0051] (1) Acetylcholinesterase and butyrylcholinesterase inhibitory activity
[0052] To the 96-well plate, 30 μL of 1.0 mmol / L thioacetylcholine iodide or thiobutyrylcholine (both purchased from Sigma), 40 μL of PBS buffer solution with pH=8.0, and 20 μL of the test compound solution ( DMSO content is less than 1%) and 10 μL acetylcholinesterase ( Ee AChE) or butyrylcholinesterase (equine serum BuChE, eq BuChE) (0.045U, all purchased from Sigma Company), after adding and mixing, incubate at 37°C for 15 min, and add 0.2% 5,5'-dithio-bis(2- Nitro) benzoic acid (DTNB, purchased from Sigma company) solution 30 μ L was developed, and the optical density value (OD value) of each well at 405 nm was measured with a microplate reader, compared with the blank well without the sample to be tested, and the compound was calculated. Enzyme inhibition rate [enzyme inhibition rate=(1-sample group OD value / blank group OD value)×100%]; select five to six concentrations of the compound, me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com