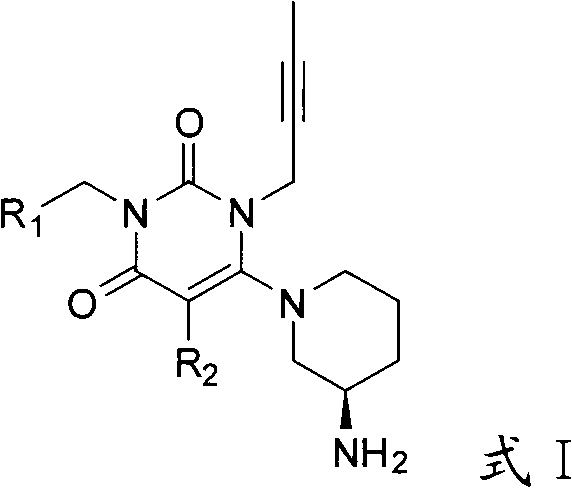
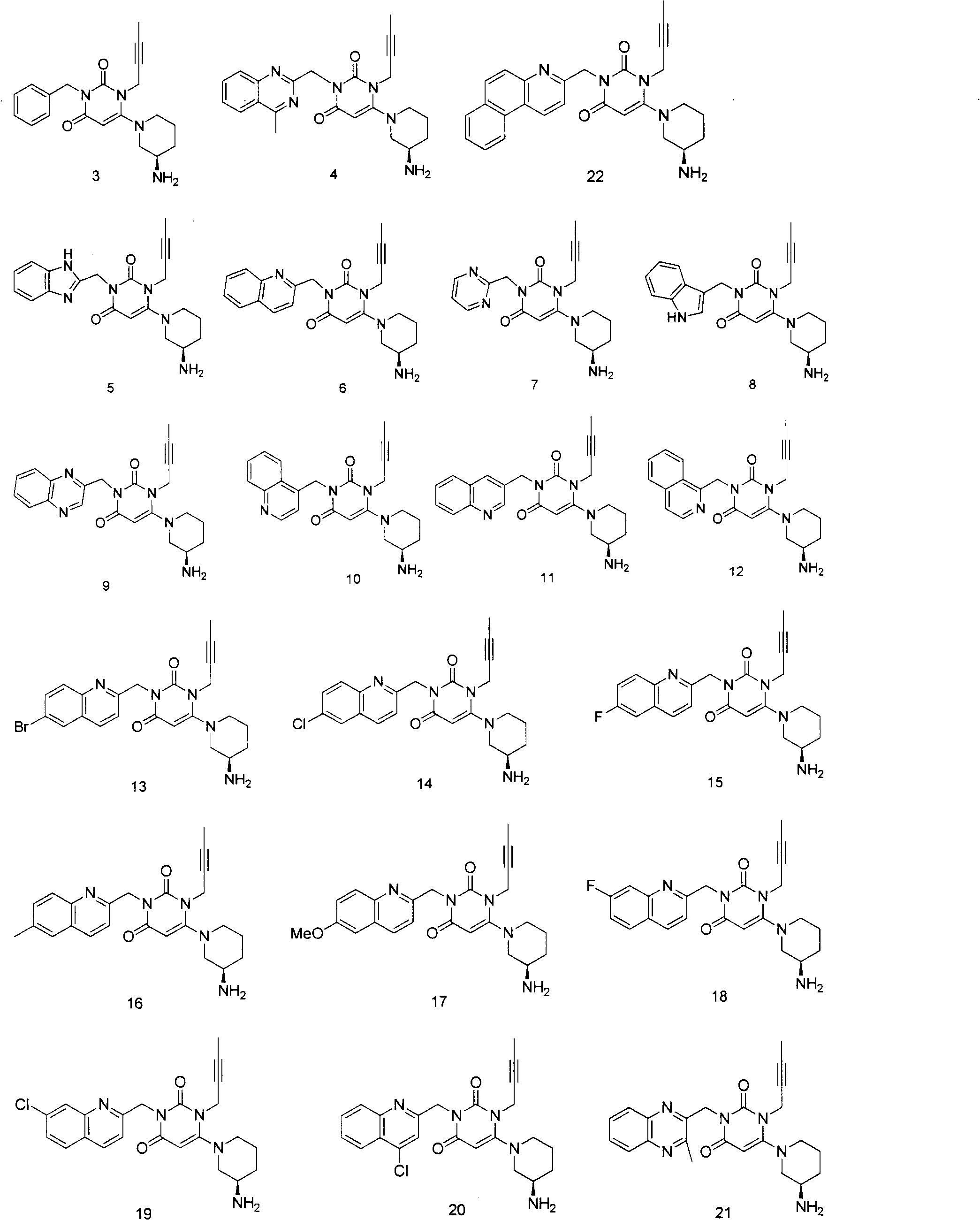
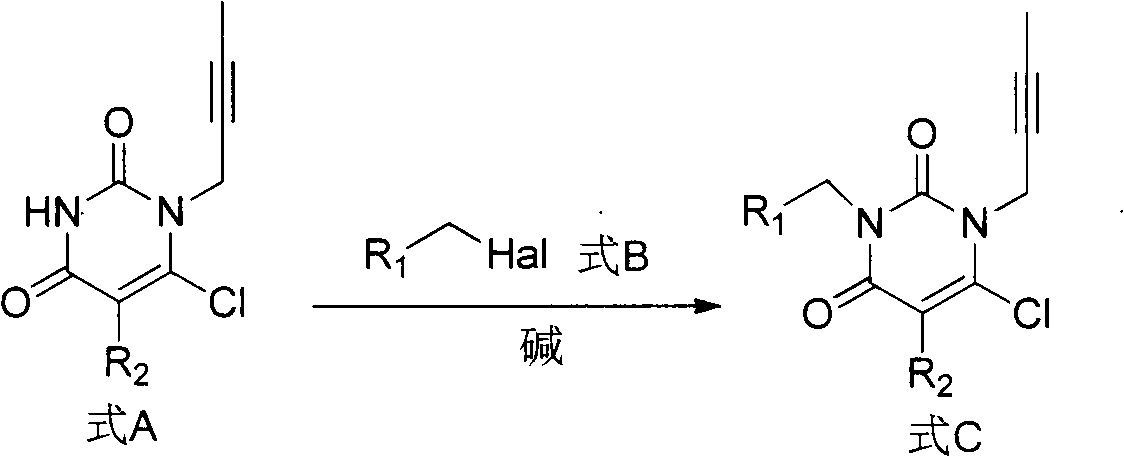
Preparation method of high-efficient DPP (dipeptidyl peptidase)-IV inhibitor
A compound, selected technology, applied in the field of medicine, can solve the problems of inactivation, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1. Synthesis of compound 4
[0089]
[0090] Synthesis of Compound 4-0:
[0091] Add 6-chlorouracil (15g, 102.4mmol) and 250mL DMF into a 500mL eggplant-shaped bottle, add 15mL DIEA after dissolving, stir evenly, add 1-bromo-2-butyne (9.85mL, 112.64mmol) dropwise, and react The solution was stirred overnight at 25°C. After the reaction is complete, add ice water, filter with suction, wash the filter cake with water and ether, and then dry it to obtain the target product.
[0092] 1H NMR (400MHz, CDCl 3 )δ8.85(s, 1H), 5.91(s, 1H), 4.75(d, J=2.0Hz, 2H), 1.82(t, J=2.4Hz, 3H). MS 199.82[M+H]+.
[0093] Synthesis of Compound 4-1:
[0094] Under nitrogen protection, 60% NaH (54mg, 3.78mmol) was added to a 100mL eggplant-shaped flask, anhydrous DME / DMF (2:1) solution was added at 0°C, and after stirring at 0°C for 10 minutes, 1-(2- Butyne)-6-chlorouracil (500mg, 2.52mmol) in 10ml of anhydrous DME / DMF (2:1) solution. After the addition was completed, anhydro...
Embodiment 2
[0099] Embodiment 2. Synthesis of compound 3
[0100]
[0101] Synthesis of compound 3-1:
[0102] Using benzyl bromide as a raw material, refer to the synthetic method of compound 4-1 in Example 1 to prepare compound 3-1.
[0103] 1 H NMR (400MHz, CDCl 3 )δ7.43-7.19(m, 5H), 5.97(s, 1H), 5.42(s, 2H), 4.60(s, 2H), 1.77(s, 3H). MS 289.07[M+H] + .
[0104] Synthesis of compound 3:
[0105] Compound 3-1 obtained in the above step was used as a raw material, and with reference to the synthetic method of compound 4 in Example 1, compound 3.
[0106] 1 H NMR (400MHz, CDCl 3 )δ7.46-7.22(m, 5H), 5.33(s, 2H), 5.20(s, 1H), 4.40(s, 2H), 3.22(m, 3H), 2.96(m, 1H), 2.62(m , 1H), 2.42(m, 1H), 1.90(m, 1H), 1.77(s, 3H), 1.60(m, 1H), 1.22(m, 1H).MS 353.2[M+H] + .
Embodiment 3
[0107] Embodiment 3. Synthesis of compound 5
[0108]
[0109] Synthesis of Compound 5-1:
[0110] Using 2-chloromethylbenzimidazole as raw material, refer to the synthetic method of compound 4-1 in Example 1 to prepare compound 5-1.
[0111] 1 H NMR (400MHz, CDCl 3 )δ7.58(m, 2H), 7.23(m, 2H), 5.99(s, 1H), 5.40(s, 2H), 4.75(d, J=2.4Hz, 2H), 1.79(t, J=2.4 Hz, 3H).MS 329.10[M+H] + .
[0112] Synthesis of Compound 5:
[0113] Compound 5-1 obtained in the above step was used as a raw material, and with reference to the synthetic method of compound 4 in Example 1, compound 5.
[0114] 1 H NMR (400MHz, CDCl 3 )δ7.47(m, 2H), 7.14(m, 2H), 5.31(s, 2H), 5.18(s, 1H), 4.40(s, 2H), 3.21(m, 3H), 2.95(m, 1H ), 2.61(m, 1H), 2.43(m, 1H), 1.92(m, 1H), 1.75(s, 3H), 1.61(m, 1H), 1.21(m, 1H). MS 393.45[M+H ] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com