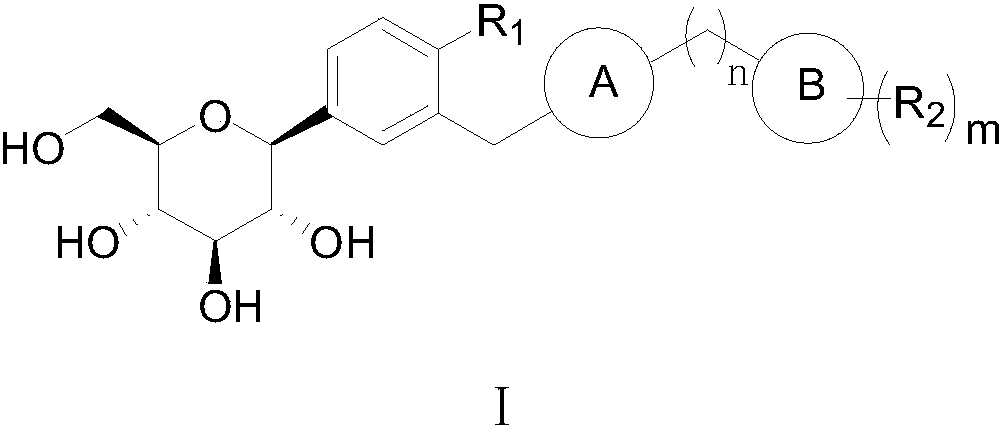
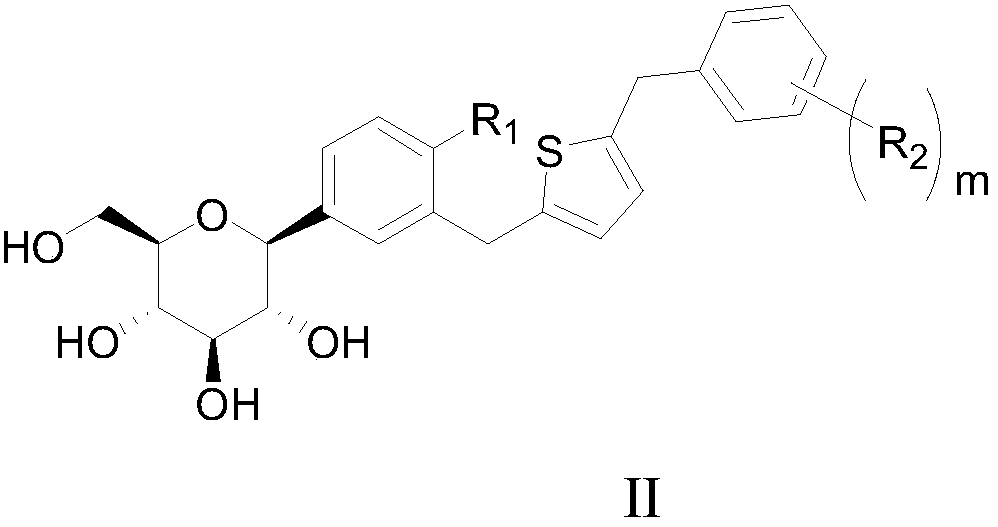
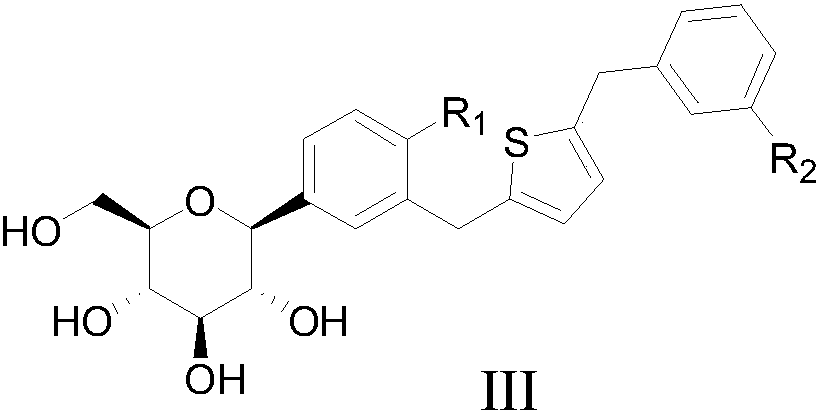
C-triaryl glucoside compound, preparation method and application of C-triaryl glucoside compound
A technology of glucoside and compound, which is applied in the field of medicine, can solve problems such as poor tolerance, weight gain, and hypoglycemia, and achieve the effects of lowering blood sugar, promoting urine sugar discharge, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1. Synthesis of compound 1
[0069]
[0070] Synthesis of compound 1-1:
[0071] Add 70.6g of 2-chloro-5-bromobenzoic acid and 500mL of tetrahydrofuran into a round bottom flask, slowly add 200mL of 2M borane dimethyl sulfide complex dropwise at 0°C; Slowly add methanol dropwise until no bubbles emerge; spin the reaction solution; then add 300mL water and 300mL ethyl acetate, extract and separate layers; wash the organic phase once with saturated brine and dry it with anhydrous sodium sulfate; The crude compound 1-1 was obtained, which was directly carried out to the next reaction without purification.
[0072] Synthesis of compound 1-2:
[0073] Add 60g PCC and 60g silica gel powder in a round bottom flask; add 500mL dichloromethane after mixing; cool to 0°C, add dropwise a dichloromethane solution (300mL) containing 45g compound 1-1 under stirring; maintain 0°C, The reaction was monitored by TLC; after the reaction was complete, the reaction solution ...
Embodiment 2
[0087] Embodiment 2. Synthesis of compound 2
[0088]
[0089] The synthetic method refers to Example 1, and the total yield is 32%.
[0090] 1 H NMR (400MHz, CDCl 3 )δ:7.24(s,1H),7.19-7.12(m,4H),6.99-6.98(m,6H),5.29(br,1H),5.07(br,1H),3.96-3.93(m,3H) ,3.77(s,2H),3.67(s,2H),3.57(s,1H),3.47(s,1H),3.35-3.33(m,2H),3.22-3.20(m,1H),2.97(br ,1H),2.87-2.81(q,J=7.2Hz,2H),1.26-1.22(t,J=7.2Hz,3H); MS m / z(ESI)537.1[M+Na] + .
Embodiment 3
[0091] Embodiment 3. Synthesis of compound 3
[0092]
[0093] The synthetic method refers to Example 1, and the total yield is 30%.
[0094] 1 H NMR (400MHz, CDCl 3 )δ:7.28(s,1H),7.18-7.08(m,4H),7.04-7.01(m,6H),5.25(br,1H),4.99(br,3H),4.02-3.91(m,4H) ,3.81(s,2H),3.69(S,2H),3.62-3.58(m,1H),3.51-3.47(m,1H),3.39-3.37(m,1H),3.23-3.21(m,1H) ,3.12(br,1H),2.85-2.80(m,1H),1.21-1.20(d,J=6.8Hz,6H); MS m / z(ESI)519.2[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com