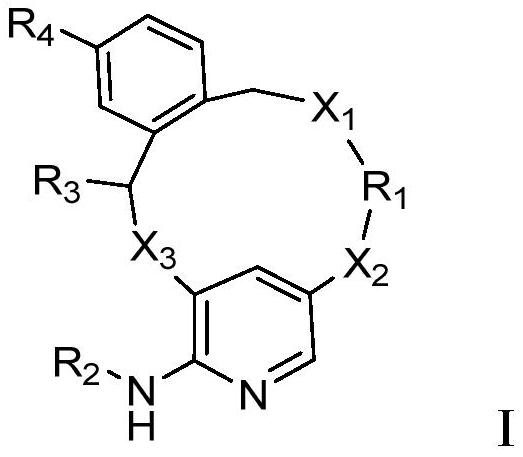
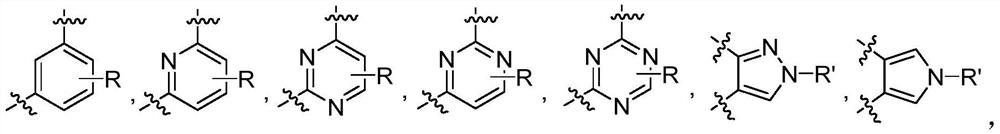
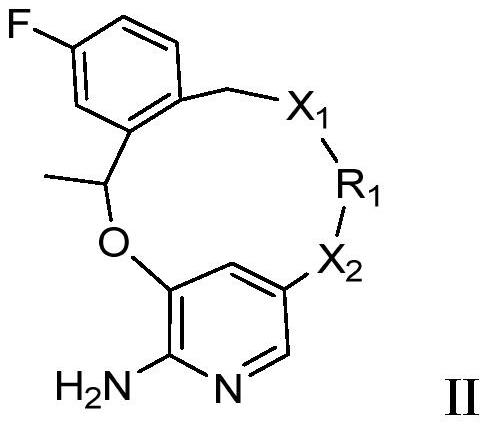
Macrocyclic JAK2 inhibitor and application thereof
A CH2, SO2 technology, applied in the direction of anti-inflammatory agents, antiviral agents, non-central analgesics, etc., can solve problems such as increased infection risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Embodiment 1 prepares compound WYL-1
[0101] 1) Synthesis of 1-(5-fluoro-2-methylphenyl)-1-ethanol
[0102]
[0103] In anhydrous THF solution of 5-fluoro-2-methylbenzaldehyde (5.00g, 0.036mol) was added dropwise 3.0M methylmagnesium bromide diethyl ether solution (13.3mL, 0.04mol) under ice-bath conditions, After the dropwise addition, the ice bath was removed, and the mixture was transferred to room temperature to react for 1.5 h, and the progress of the reaction was monitored by TLC. After the reaction is complete, use saturated NH 4 Aqueous Cl solution quenched the reaction. The reaction solution was extracted three times with ethyl acetate and the organic phases were combined, dried over anhydrous sodium sulfate and evaporated under reduced pressure to obtain 5.03 g (90.13%) of a colorless oily liquid.
[0104] 1 H NMR (400MHz, CDCl 3 )δ7.15(dd, J=10.2Hz, 2.8Hz, 1H), 6.99(dd, J=8.3Hz, 5.8Hz, 1H), 6.78-6.75(m, 1H), 5.03-4.95(m, 1H) ,2.20(s,3H),1.84(s,1H),1...
Embodiment 2
[0129] Embodiment 2 prepares compound WYL-2
[0130]
[0131] WYL-2 was synthesized by the method of synthesizing WYL-1 to obtain 23 mg (3.76%) of light red solid.
[0132] mp:249.8-251.5℃. 1 H NMR (400MHz, CDCl 3 )δ7.46(d, J=1.6Hz, 1H), 7.40(d, J=1.8Hz, 1H), 7.33(dd, J=8.7Hz, 5.9Hz, 1H), 7.17(dd, J=13.1, 4.6Hz, 2H), 6.94-6.89(m, 1H), 5.36-5.30(m, 1H), 4.74(s, 2H), 4.37(d, J=15.7Hz, 1H), 4.07(d, J=15.6 Hz,1H),3.67(s,3H),1.67(d,J=6.5Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ162.44,160.03,155.17,150.39,140.39,138.82,131.82,128.20,128.00,117.89,115.37,112.39,107.38,75.15,49.07,45.30,38.43,21.90:( +H) + calcd for C 18 h 19 FN 5 O, 340.1574; found 3340.1575. HPLC purity: 96.7%, retention time = 13.15min.
Embodiment 3
[0133] Embodiment 3 prepares compound WYL-3
[0134] 1) Synthesis of (4-hydroxypyrimidin-2-yl) tert-butyl carbamate
[0135]
[0136] Dissolve 2-aminopyrimidin-4-ol (1.00g, 0.01mmol) in 20mL of pyridine, and add (Boc) dropwise to the reaction solution at 65°C 2 O (2.94g, 0.014mol), after the dropwise addition, the temperature was raised to 85°C for 4h, and the reaction progress was monitored by TLC. After the reaction was completed, the reaction solution was extracted with EA, the organic phase was washed with acid water, and passed through a silica gel column to obtain 1.06 g (53.65%) of a white solid.
[0137] 1 H NMR (400MHz, DMSO-d 6 )δ11.28(s,2H),7.70(d,J=6.8Hz,1H),5.93(d,J=6.8Hz,1H),1.48(s,9H).LC-MS(ESI):m / z:212.1(M+H) + .
[0138] 2) 4-((2-(1-((5-bromo-2-nitro-pyridin-3-yl)oxy)ethyl)-4-fluorobenzyl)oxy)pyrimidin-2-amine synthesis
[0139]
[0140] 5-Bromo-3-(1-(2-(bromomethyl)-5-fluorophenyl)ethoxy)-2-nitropyridine (0.11g, 0.25mmol), (4-hydroxypyrimidine-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com