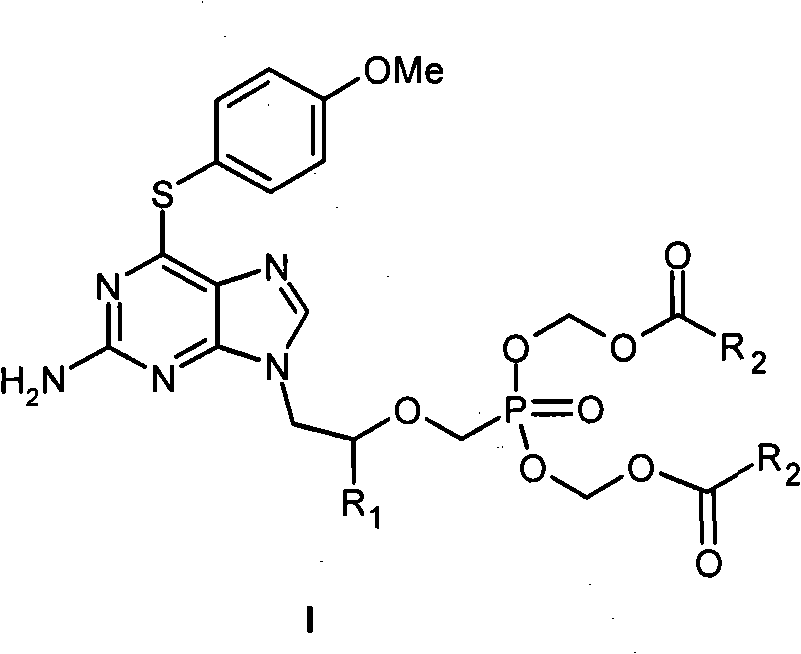
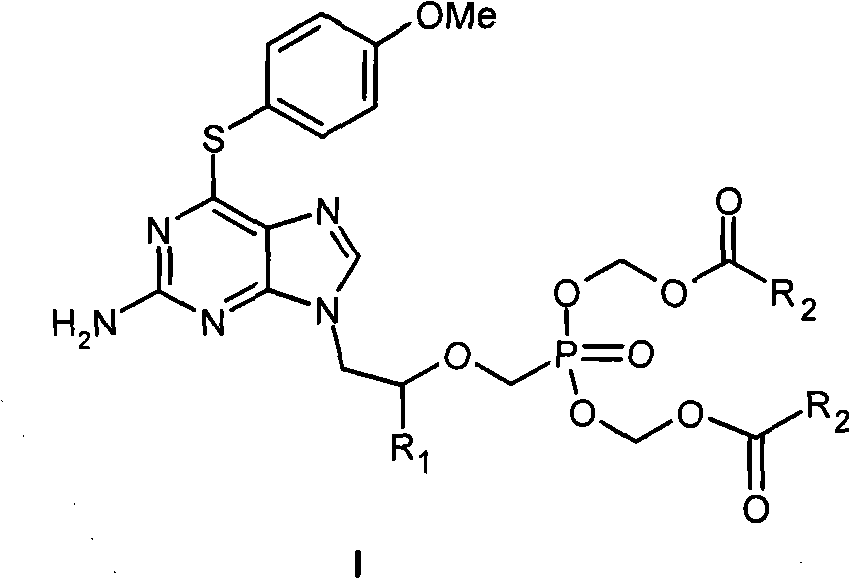
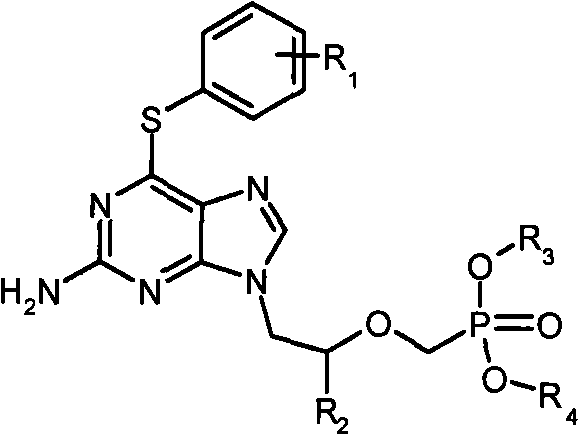
Acyclic nucleoside phosphonate derivative and medicinal application thereof
一种核苷膦酸酯、衍生物的技术,应用在医药配方、含有效成分的医用配制品、药物组合等方向,能够解决没有作用等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0128] The present invention can be further described by the following examples, however, the scope of the present invention is not limited to the following examples. Those skilled in the art can understand that various changes and modifications can be made in the present invention without departing from the spirit and scope of the present invention.
[0129] The present invention provides general and / or specific descriptions of the materials and test methods used in the tests. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible.
Embodiment 1
[0130] The preparation of embodiment 1,2-(diisopropyl-phosphonomethoxy)-ethyl chloride The synthetic route is as follows:
[0131]
[0132] In a 150ml three-neck flask, add 84.6g (70.5ml, 1.05mol) of 2-chloroethanol and 31.6g (1.09mol) of pulverized paraformaldehyde, and pass in dry hydrogen chloride gas under stirring for 24 hours. After the stirring was stopped, the reaction solution was divided into two layers; the lower layer was separated and dried with calcium chloride. After filtration, the filtrate was fractionated under reduced pressure, and the fraction with a boiling range of 80-84°C / 28-30mmHg was collected to obtain 74.3 g of chloromethyl-2-chloroethyl ether.
[0133] In a 300 mL reaction flask, add 18 g (0.3 mol) of isopropanol, 23.7 g (0.3 mol) of pyridine and 100 ml of petroleum ether, and cool in an ice bath. A solution of 13.8g (0.1mol) phosphorus trichloride in 40ml petroleum ether was added dropwise under vigorous stirring. After the addition, stir and...
Embodiment 2
[0135] Example 2, (R)-{1-methyl-2-[(1-methylsulfonyloxy)ethoxy]methyl}phosphonic acid Preparation of diisopropyl ester
[0136] The synthetic route is as follows:
[0137]
[0138] Add 24.0g (0.315moL) (R)-1,2-propanediol, 0.25g N,N-dimethylaminopyridine, 350mL dichloromethane and 46.0g (0.45moL) triethylamine in a 1000ml flask, and cool in an ice-water bath ,Magnetic stirring. Add 88.7g (0.315moL) triphenylchloromethane in batches, and add it in about 1 hour, remove the ice-water bath in 1.5 hours, and react for about 15 hours after rising to room temperature (TLC detection, until the triphenylchloromethane disappears). Add distilled water (100mL) to dissolve the triethylamine hydrochloride generated by the reaction, transfer to a separatory funnel for layering, and wash the oil phase with 5% sodium bicarbonate solution (2×100mL), distilled water (100mL) successively, and wash with anhydrous sulfuric acid Sodium-dried, filtered, and the filtrate was distilled off the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com