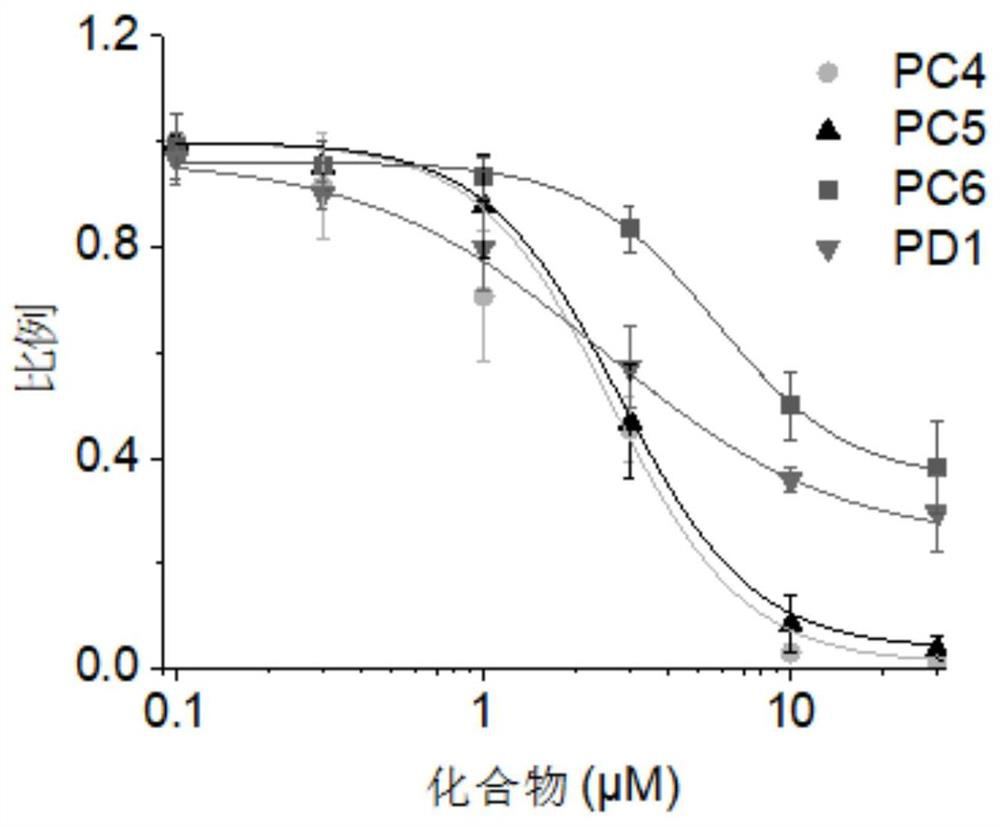
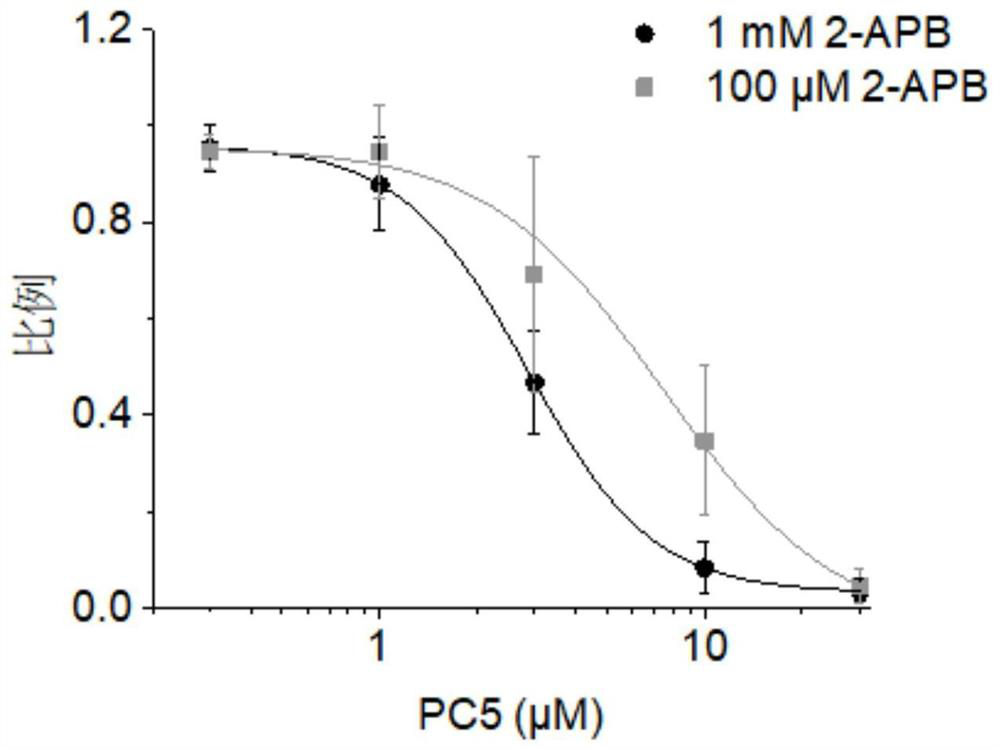
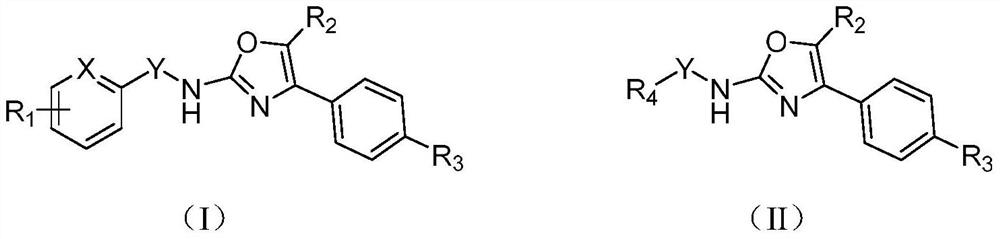
TRPV3 small molecule allosteric inhibitor and preparation method thereof
A technology of allosteric inhibitors and small molecules, which is applied in the field of compounds and their preparation, can solve the problems of slow development, large effective dose, and few structural types, and achieve the effect of increasing structural types and promoting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of 2,6-dimethoxy-N-(4-(4-(trifluoromethyl)phenyl)oxazol-2-yl)benzamide:
[0050]
[0051] Step 1: Simultaneously add 2,6-dimethoxybenzoic acid (1.76mmol, 2.0eq.) and excess thionyl chloride, reflux at 85°C for 1-2h, and monitor the reaction by thin-layer chromatography until the raw material disappears. The excess thionyl chloride was evaporated under reduced pressure to obtain a solid.
[0052] Step two:
[0053] Method 1: Put IB 1 (0.88mmol, 1.0eq.) was dissolved in THF, and TEA (2.64mmol, 3.0eq.) was added. The solid obtained in Step 1 (1.76mmol, 2.0eq.) was dissolved in THF under the condition of ice bath and added dropwise to the mixture. After reacting for 1h, the temperature was raised to 75°C and refluxed overnight. The reaction was monitored by TLC. After the reaction was completed, the reaction mixture was cooled to room temperature, and an appropriate amount of saturated NaHCO was slowly added thereto. 3 The solution was quenched and extracte...
Embodiment 2
[0056] Synthesis of 2,3-dimethoxy-N-(4-(4-(trifluoromethyl)phenyl)oxazol-2-yl)benzamide:
[0057]
[0058] The synthetic method of reference compound (I-1), white solid, the yield is 80%. 1 H NMR (400MHz, DMSO-d 6 )δ8.07(s,1H),7.90(s,2H),7.78(dd,J=49.2,8.2Hz,2H),7.53–7.39(m,1H),7.29(t,J=8.1Hz,1H ),6.84(s,1H),3.87(d,J=21.6Hz,6H).HRMS(ESI):calcd.for C 19 h 15 f 3 N 2 o 4 (M+H) + 393.1057; found 393.1046.
Embodiment 3
[0060] Synthesis of 2,4-dimethoxy-N-(4-(4-(trifluoromethyl)phenyl)oxazol-2-yl)benzamide:
[0061]
[0062] Referring to the synthetic method of compound (I-1), method 1 in step 2 was adopted, and the yield was 26% as a pale yellow solid. Method 2 in step 2 can be used, and the yield will be improved. 1 H NMR (400MHz, DMSO-d 6 )δ10.83(s,1H),8.64(s,1H),7.98(d,J=8.1Hz,2H),7.81(d,J=8.2Hz,2H),7.76(d,J=8.6Hz, 1H),6.72–6.67(m,2H),3.90(d,J=30.6Hz,6H).HRMS(ESI):calcd.for C 19 h 15 f 3 N 2 o 4 (M+H) + 393.1057; found 393.1048.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com