Chiral ferrocene diphosphine ligand and preparation method thereof
A technology of dichlorination and compound is applied in the field of chiral ferrocene bisphosphine ligand and its preparation, which can solve the problems such as being difficult to obtain, and achieve the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
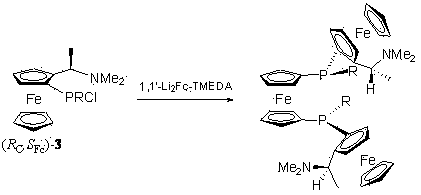
[0042] [( R C , R C ),( S Fc , S Fc ),( S P , S P )]-1,1′-bis[2-(1- N , N -Dimethylaminoethyl)-1-ferrocenyl] the preparation of phenylphosphinoferrocene (Trifer)
[0043] Towards( R )-Ugi′s amine (2.57 g, 10 mmol) was injected with 20 mL of dry tert-butyl methyl ether (TBME), placed in an ice-water bath, and 1.3 M of t -BuLi (8.5 mL, 11.05 mmol), after the dropwise addition, the reaction was raised to room temperature for 1.5 hours; the reaction solution was placed in a low-temperature reactor at -78°C, and PCl dissolved in 5 mL TBME was slowly added dropwise below -78°C 3 (1 mL, 11.46 mmol), after the dropwise addition, the reaction was raised to room temperature for 1.5 hours to generate dichloro 2; Place the reaction below -78°C again, slowly add the suspension of phenyllithium [bromobenzene (1.75 g, 11.1 mmol) at -40°C at 1.6 M n -BuLi (7.6 mL, 12.16mmol) was prepared by lithiation for 2 hours] After the addition, the reaction was raised to room temperatur...
Embodiment 2
[0045] [( R C , R C ),( S Fc , S Fc ),( S P , S P )]-1,1′-bis[2-(1- N , N -Dimethylaminoethyl)-1-ferrocenyl]cyclohexylphosphinoferrocene (Cy-Trifer) preparation
[0046] The preparation method is the same as that of Example 1, and the suspension of phenyllithium is replaced with a THF solution (11 mL, 11 mmol) of 1.0 MCyMgBr to obtain 2.08 g of the product Cy-Trifer, with a yield of 45%; m.p.: 182.5-185.5oC; [ α] = -302.9 (c = 0.25, CH 2 Cl 2 ); 1 H NMR (400 Hz, CDCl 3 ) δ4.67 (s, 2H), 4.50 (d, J = 9.0 Hz, 4H), 4.31 (s, 2H), 4.24 (s, 2H), 4.10-3.99 (m, 4H), 3.93 (s, 10H), 3.69 (s, 2H), 2.42-2.27 (m, 2H), 2.13 (s, 12H), 2.03-1.92 (m, 2H), 1.86-1.57 (m, 11H), 1.36-1.16 (m, 6H), 1.27 (d, J = 6.6 Hz, 7H); 31 P NMR (162 Hz, CDCl 3 ) δ -26.25 (s); 13 C NMR (101 Hz, CDCl 3 ) δ96.35 (d, J = 22.9 Hz), 79.61 (d, J = 8.1 Hz), 78.92 (d, J = 16.1 Hz), 76.46 (d, J = 33.2 Hz), 72.00 (d, J = 5.5 Hz), 71.76 (d, J = 4.9 Hz), 70.97 (d, J = 7.5 Hz), 69.52 ...
Embodiment 3
[0048] ( R C , S Fc , S P )-1-[2-(1- N , N Preparation of -Dimethylaminoethyl)-1-ferrocenyl]phenylphosphino-1'-dicyclohexylphosphinoferrocene (ChenPhos)
[0049] monochloride 3 The preparation is the same as that in Example 1. The reaction solution is placed in a low-temperature reactor at -78°C, and 1-bromo-1'-lithium ferrocene [below -20°C, 1,1'-dibromobis Ferrocene (3.76 g, 11 mmol) and 1.6 M n -BuLi (7.6 mL, 12.16mmol) was prepared by reacting in THF for 2 hours], after the addition, the reaction was raised to room temperature and reacted for 1.5 hours to generate an intermediate 6 , place the reaction solution below -60°C, add dropwise 1.3 M t -BuLi (8.5 mL, 11.05mmol), reacted at -60°C for 2 hours after the dropwise addition, slowly added Cy 2 A solution of PCl (2.7 mL, 12.2 mmol) in 5 mL of dry TBME was added dropwise and then raised to room temperature to react overnight. The reaction was quenched by adding saturated ammonium chloride solution, the organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com