Method for catalytic oxidation of cycloalkane by confinement porphyrin Co (II)
A technology for catalytic oxidation and cycloalkane, which is applied in chemical instruments and methods, oxidation reaction preparation, organic compound preparation, etc., to achieve enhanced stability, improved conversion rate, and beneficial separation of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
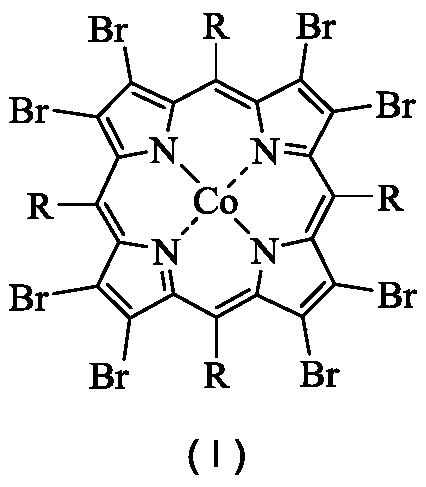
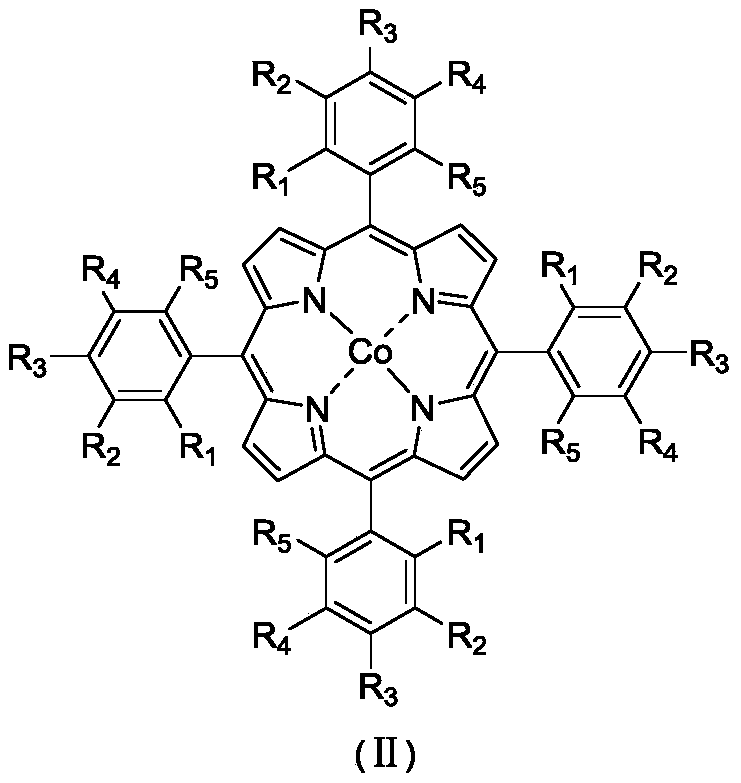
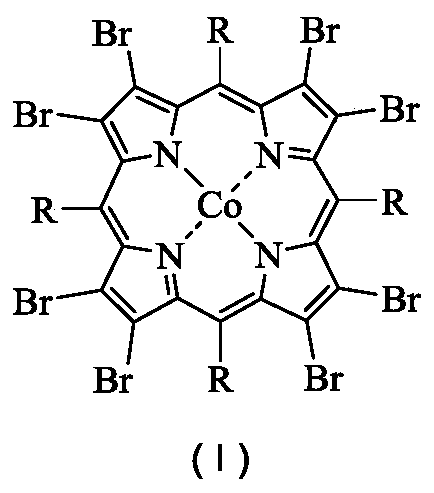
[0036] In a 100mL stainless steel autoclave with polytetrafluoroethylene liner, 0.00026g (0.0002mmol) 5,10,15,20-tetraphenyl-2,3,7,8,12,13,17,18 - Octabromoporphyrin cobalt (II) was dispersed in 16.8320 g (200 mmol) of cyclohexane, the reaction vessel was sealed, the temperature was raised to 120° C. with stirring, and oxygen was introduced to 1.0 MPa. At 120°C, 1.0MPa oxygen pressure, 800rpm stirring reaction for 8.0h. After completion of the reaction, cool to room temperature with ice water, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 30min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 100 mL. Pipette 10 mL of the resulting solution, and use toluene as an internal standard for gas chromatography analysis; pipette 10 mL of the resulting solution, and use benzoic acid as an internal standard for liquid chromatography analysis. Cyclohexane conversion 5.53%, cyclohexanol selec...
Embodiment 2
[0038] In a 100mL stainless steel autoclave with polytetrafluoroethylene liner, 0.0026g (0.002mmol) 5,10,15,20-tetraphenyl-2,3,7,8,12,13,17,18 - Octabromoporphyrin cobalt (II) was dispersed in 16.8320 g (200 mmol) of cyclohexane, the reaction vessel was sealed, the temperature was raised to 120° C. with stirring, and oxygen was introduced to 1.0 MPa. At 120°C, 1.0MPa oxygen pressure, 800rpm stirring reaction for 8.0h. After completion of the reaction, cool to room temperature with ice water, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 30min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 100 mL. Pipette 10 mL of the resulting solution, and use toluene as an internal standard for gas chromatography analysis; pipette 10 mL of the resulting solution, and use benzoic acid as an internal standard for liquid chromatography analysis. Cyclohexane conversion 5.72%, cyclohexanol selecti...
Embodiment 3
[0040] In a 100mL stainless steel autoclave with polytetrafluoroethylene liner, 0.0261g (0.0200mmol) 5,10,15,20-tetraphenyl-2,3,7,8,12,13,17,18 - Octabromoporphyrin cobalt (II) was dispersed in 16.8320 g (200 mmol) of cyclohexane, the reaction vessel was sealed, the temperature was raised to 120° C. with stirring, and oxygen was introduced to 1.0 MPa. At 120°C, 1.0MPa oxygen pressure, 800rpm stirring reaction for 8.0h. After completion of the reaction, cool to room temperature with ice water, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 30min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 100 mL. Pipette 10 mL of the resulting solution, and use toluene as an internal standard for gas chromatography analysis; pipette 10 mL of the resulting solution, and use benzoic acid as an internal standard for liquid chromatography analysis. Cyclohexane conversion 5.76%, cyclohexanol select...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com