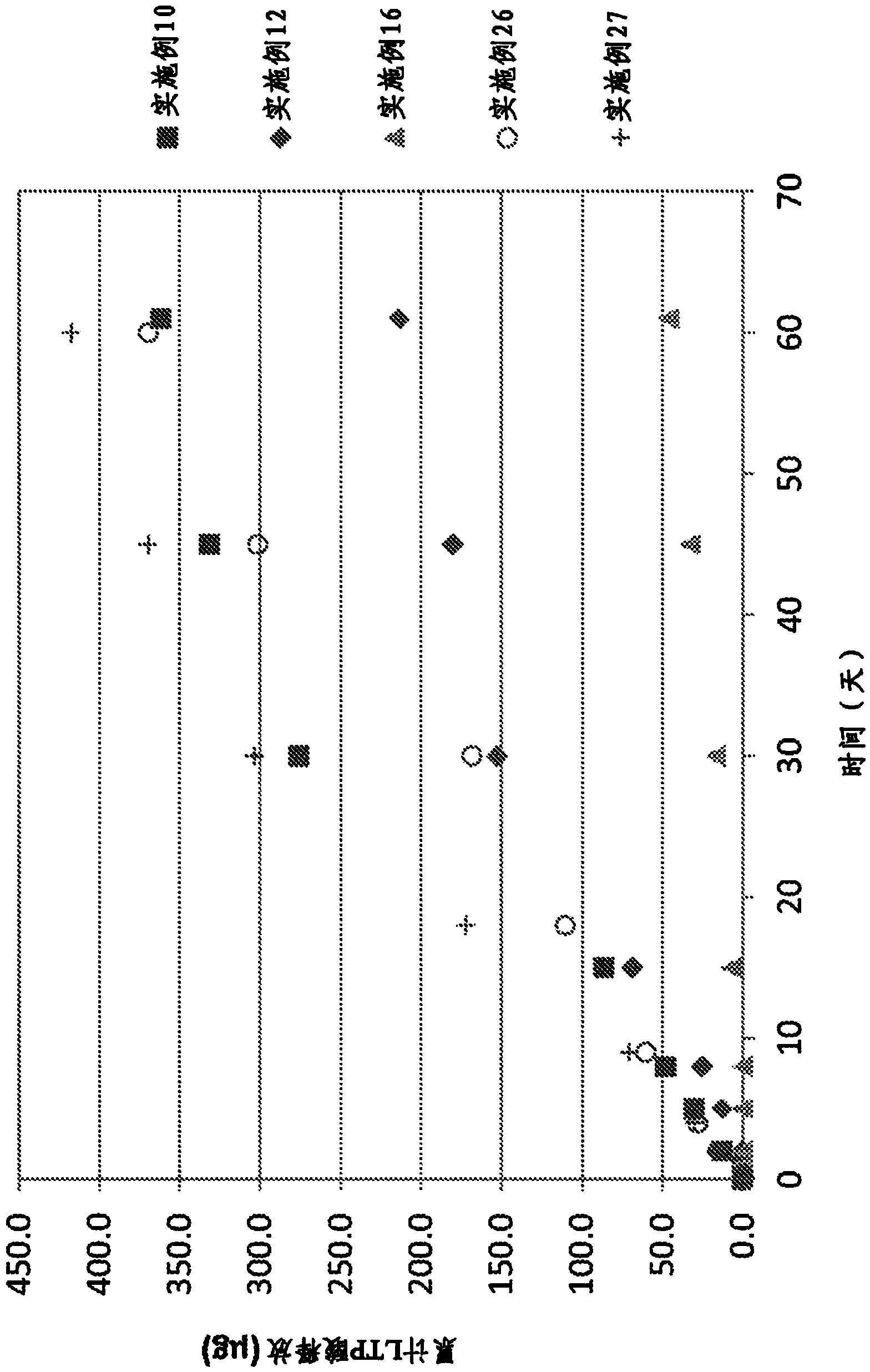
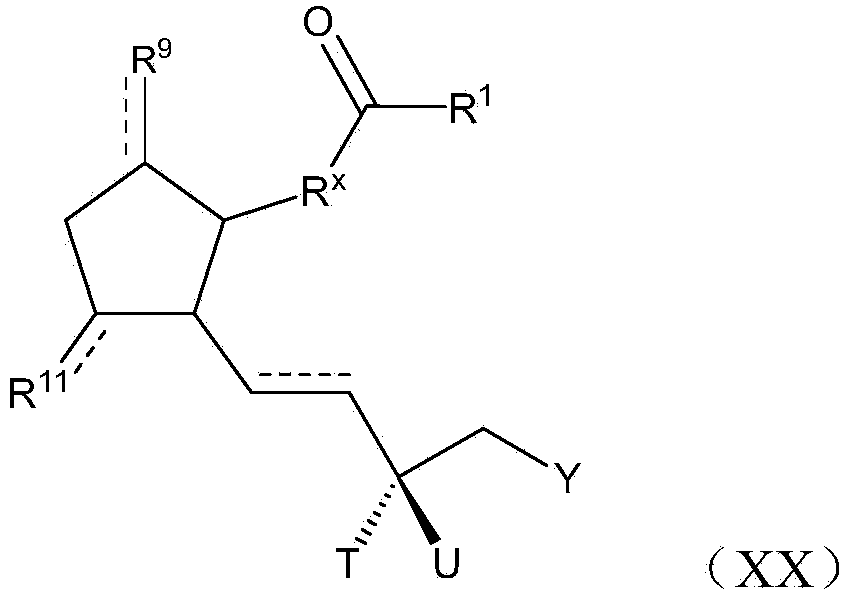
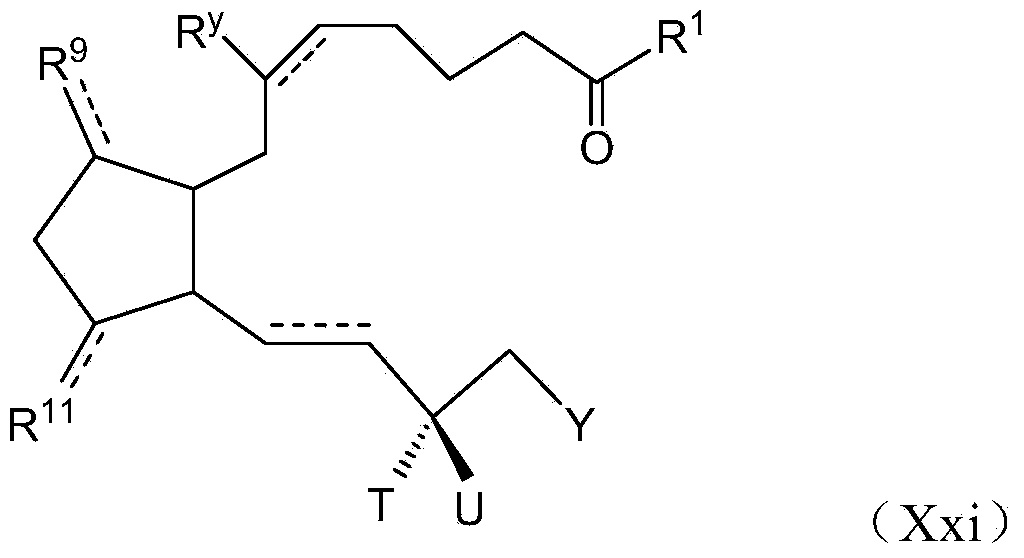
Polymer conjugated prostaglandin analogues
A prostaglandin and polymer technology, which is applied in the direction of drug combination, eicosanoid active ingredients, medical preparations of non-active ingredients, etc., can solve the problems of patient compliance and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0697] (Z)-3-Hydroxy-2-(hydroxymethyl)-2-methylpropyl 7-((1R,2R,3R,5S)-3,5-dihydroxy-2-((R)-3 -Hydroxy-5-phenylpentyl)cyclopentyl)hept-5-enoate (2)
[0698]
[0699]Following the general procedure for HBTU coupling (Procedure 1), use latanoprost free acid (1) (407.1 mg, 1.0 mmol), HBTU (440.3 mg, 1.2 mmol), 1,1,1-trihydroxymethyl A solution of ethyl ethane (187.9 mg, 1.6 mmol) and triethylamine (0.60 mL, 4.3 mmol) in anhydrous THF. Chromatography of the residue (SiO 2 , MeOH-CHCl 3 , 10:90), the title compound (2) (322.0 mg, 63% yield) was obtained as a colorless clear oil. ESI-MS: m / z 538 ([M+2Na] + ); 1 H NMR (400MHz, CDCl 3 ) δ (ppm): 7.34-7.16 (m, 3H), 7.16-7.00 (m, 2H), 5.43-5.36 (m, 1H), 5.35-5.18 (m, 1H), 4.16-3.97 (m, 2H) , 3.89-3.74 (m, 1H), 3.61-3.51 (m, 1H), 3.45 (s, 3H), 3.41-3.31 (m, 4H), 2.80-2.65 (m, 2H), 2.65-2.46 (m, 2H), 2.40-1.96 (m, 5H), 1.91-1.35 (m, 8H), 1.35-1.20 (m, 2H), 0.77 (s, 2H).
Embodiment 2
[0700] Example 2 (Z)-1,3-dihydroxypropan-2-yl 7-((1R,2R,3R,5S)-3,5-dihydroxy-2-((R)-3-hydroxyl-5 -Phenylpentyl)cyclopentyl)hept-5-enoate (5)
[0701]
[0702] Following the general procedure for HBTU coupling (Procedure 1), use latanoprost free acid (1) (528.2 mg, 1.35 mmol), 1,3-benzylidene glycerol (309.0 mg, 1.71 mmol), HBTU ( 564.5mg, 1.49mmol), and triethylamine (0.8ml, 5.75mmol) in anhydrous DCM. Chromatographic analysis of the crude material (SiO 2 , EtOAc, 100%), the benzylidene ester (3) was obtained as a colorless clear oil (412.3 mg, 55% yield). ESI-MS: m / z 575 ([M+Na] + ); 1 H NMR (400MHz, CDCl 3 ) δ (ppm): 7.49-7.37 (m, 2H), 7.37-7.24 (m, 3H), 7.24-7.16 (m, 2H), 7.16-7.03 (m, 3H), 5.48 (s, 1H), 5.41 -5.31 (m, 4H), 4.70-4.57 (m, 1H), 4.26-3.94 (m, 5H), 3.90-3.69 (m, 1H), 3.81-3.82 (m, 1H), 2.77-2.64 (m, 1H), 2.62-2.54 (m, 1H), 2.38 (td, J=7.2, 1.2Hz, 3H), 2.30-1.98 (m, 6H), 1.82-1.35 (m, 10H), 1.35-1.13 (m, 2H).
[0703] Following the general procedure ...
Embodiment 3
[0705] 1,3-dihydroxypropan-2-yl 4-(((Z)-7-((1R,2R,3R,5S)-3,5-dihydroxy-2-((R)-3-hydroxy- 5-phenylpentyl)cyclopentyl)hept-5-enoyl)oxy)benzoate (6)
[0706]
[0707] Following the general procedure for HBTU coupling (Procedure 1), use latanoprost free acid (1) (234.1 mg, 0.60 mmol), 2-phenyl-1,3-dioxan-5-yl 4-hydroxy A solution of benzoate (361.5mg, 1.20mmol), HBTU (251.4mg, 0.66mmol) and triethylamine (0.5ml, 3.59mmol) in anhydrous DCM (15ml). Chromatographic analysis of the crude material (SiO 2 , EtOAc, 100%), the benzylidene ester (4) was obtained as a colorless clear oil (258.7 mg, 63% yield). ESI-MS: m / z695 ([M+Na] + ); 1 H NMR (400MHz, CDCl 3 ) δ (ppm): 8.17-8.04 (m, 2H), 7.55-7.40 (m, 2H), 7.40-7.25 (m, 3H), 7.25-7.16 (m, 2H), 7.16-7.02 (m, 5H) , 5.55 (s, 1H), 5.50-5.26 (m, 2H), 4.94-4.79 (m, 1H), 4.41-4.12 (m, 4H), 4.12-3.97 (m, 1H), 3.93-3.79 (m, 1H), 3.65-3.49 (m, 1H), 2.73-2.55 (m, 2H), 2.43-2.06 (m, 5H), 1.87-1.38 (m, 13H), 1.38-1.22 (m, 2H).
[0708] Fol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com