Polynucleated megakaryocytic cell and method for manufacturing platelets
A technology for megakaryocytes and platelets, applied in the field of platelet manufacturing, can solve the problem of non-muscle type II myosin that cannot be observed locally, and achieve the effects of promoting multinucleation, increasing the number, and shortening the time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
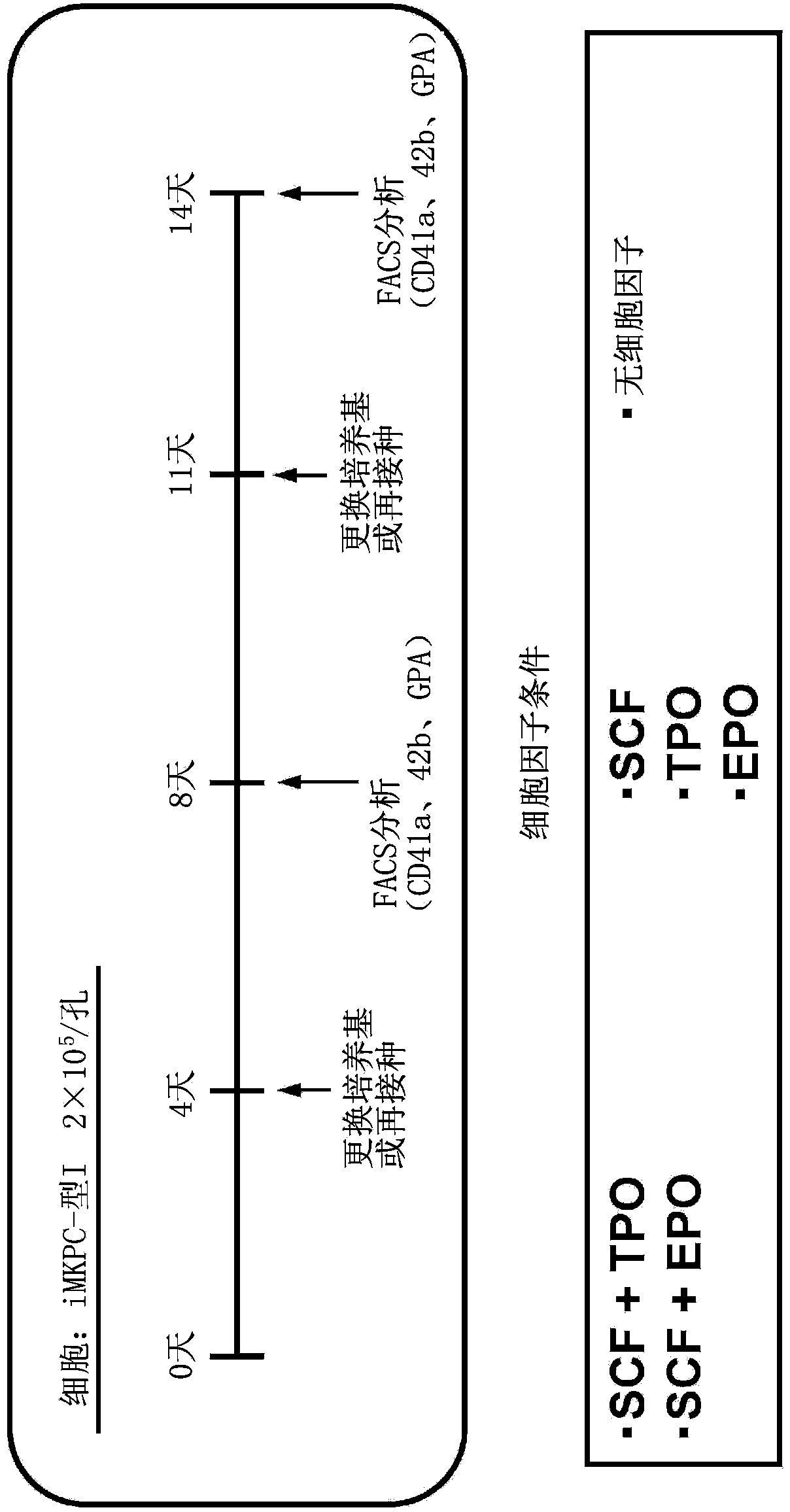
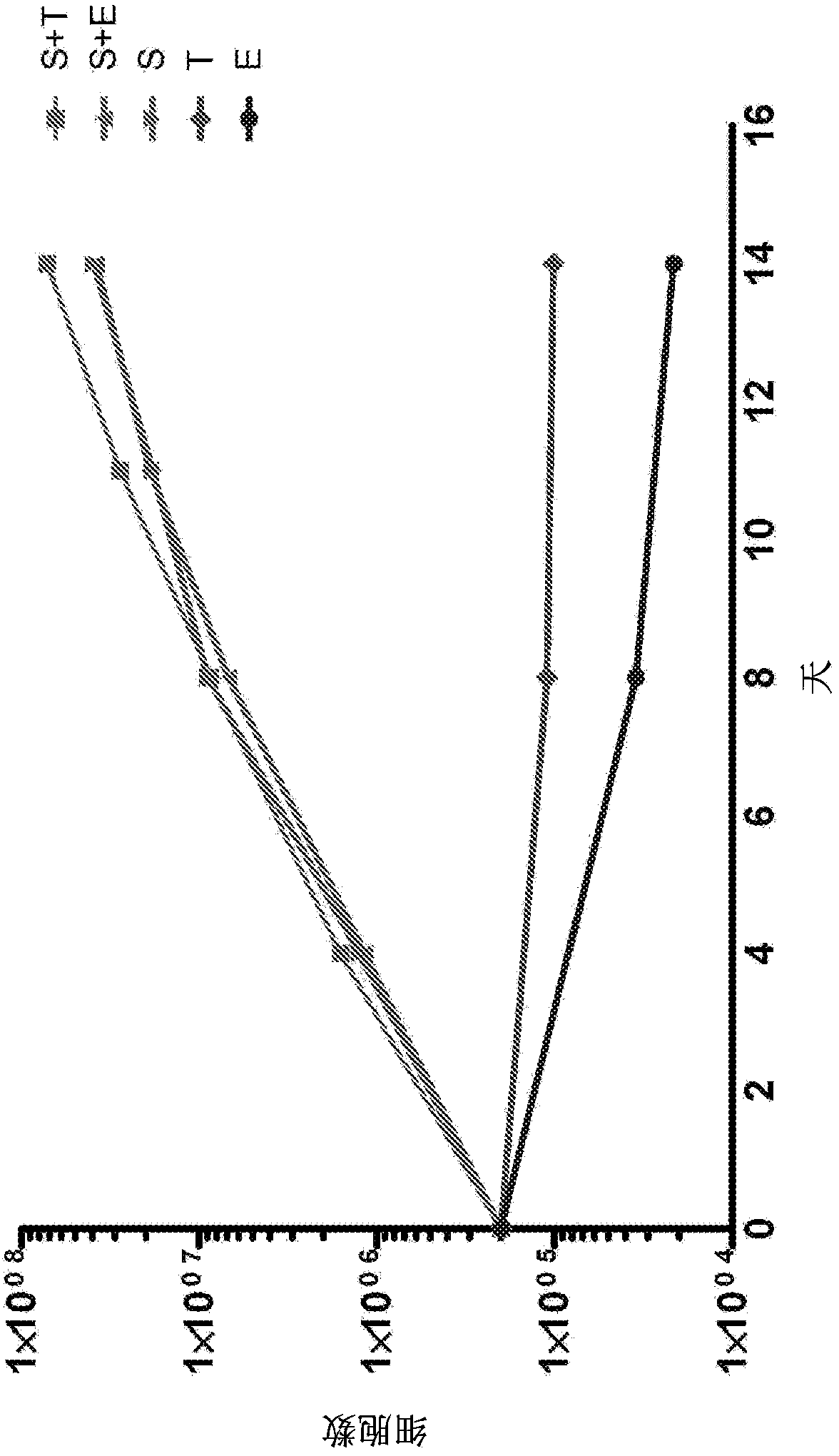
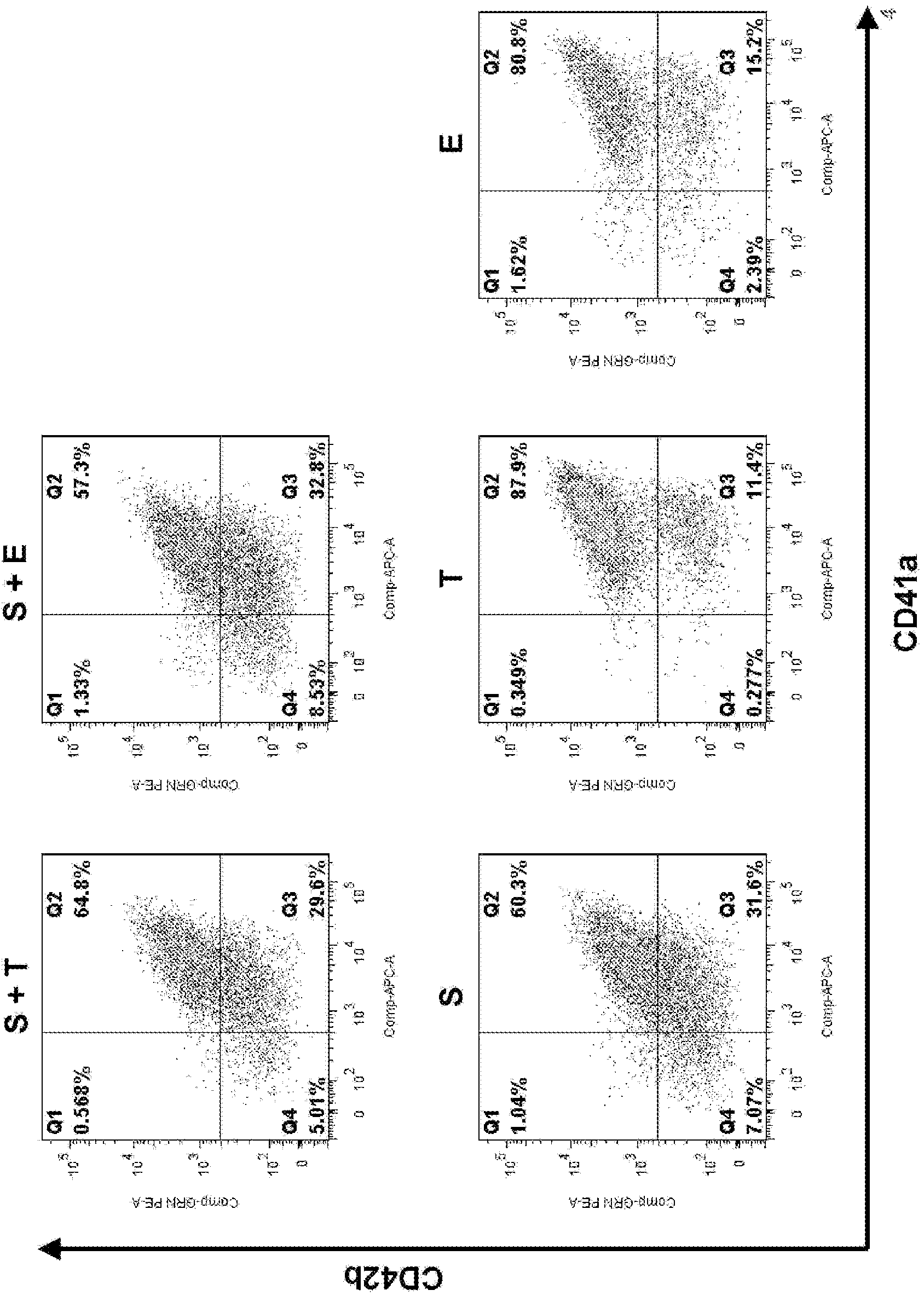
[0171] 1. Preparation of Megakaryocytes Before Multinucleation
[0172] 1-1. Preparation of pre-multinucleated megakaryocytes from ES cells
[0173] In order to study the multinucleation of megakaryocytes, megakaryocytes before multinucleation were prepared from ES cells (see Patent Document 3 for details).
[0174] The human ES cell line [KhES-3] was cultured in the presence of 20ng / ml VEGF for 14 days to prepare a network structure, and the hematopoietic precursor cells removed from the network structure were recovered and planted on 10T1 / 2 cells to Reach cell number 1×10 5 / hole.
[0175] The hematopoietic precursor cells thus prepared were infected with pMx tet off c-MYC2A BMI1 retroviral vector integrating c-MYC-2A-BMI1 three times every 12 hours at MOI=10 (confirmed by Jurkat cells) to perform c-MYC and induction of BMI1 expression (Patent Document 3). The pMx tet off c-MYC2A BMI1 vector can express the c-MYC gene and BMI1 gene in the presence of estradiol, while...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com