Ziconotide and impurity detection method
A technology of ziconotide and impurities, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of large polarity difference, difficulty in effective separation, waste of manpower and material resources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Instrument conditions
[0055] High performance liquid chromatography: Waters2695
[0056] Chromatographic column: Kromasil C18 (250mm×4.6mm);
[0057] Flow rate: 1.0ml / min
[0058] Column temperature: 40°C
[0059] Injection volume: 20μl
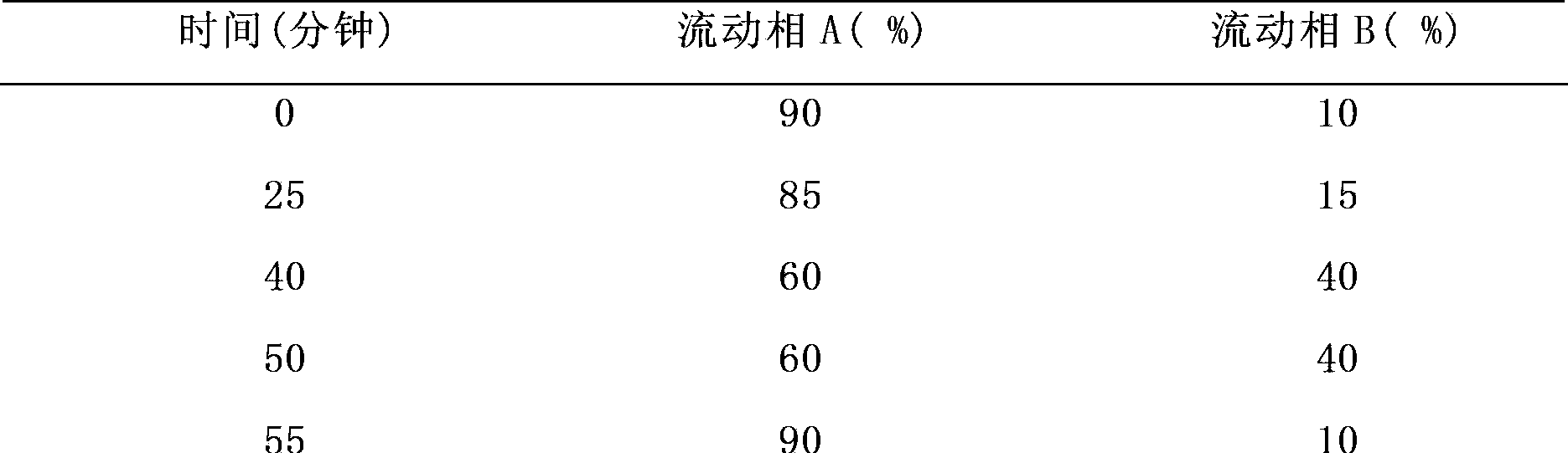
[0060] Mobile phase: mobile phase A is 0.01M potassium dihydrogen phosphate aqueous solution: acetonitrile = 90:10 (after the buffer solution and acetonitrile are fully mixed, adjust the pH to 6.5 with triethylamine), mobile phase B is 0.01M potassium dihydrogen phosphate aqueous solution: Acetonitrile (after the buffer solution and acetonitrile are fully mixed, adjust the pH to 6.5 with triethylamine) = 50:50, the gradient of the mobile phase is shown in Table 3 below.
[0061]
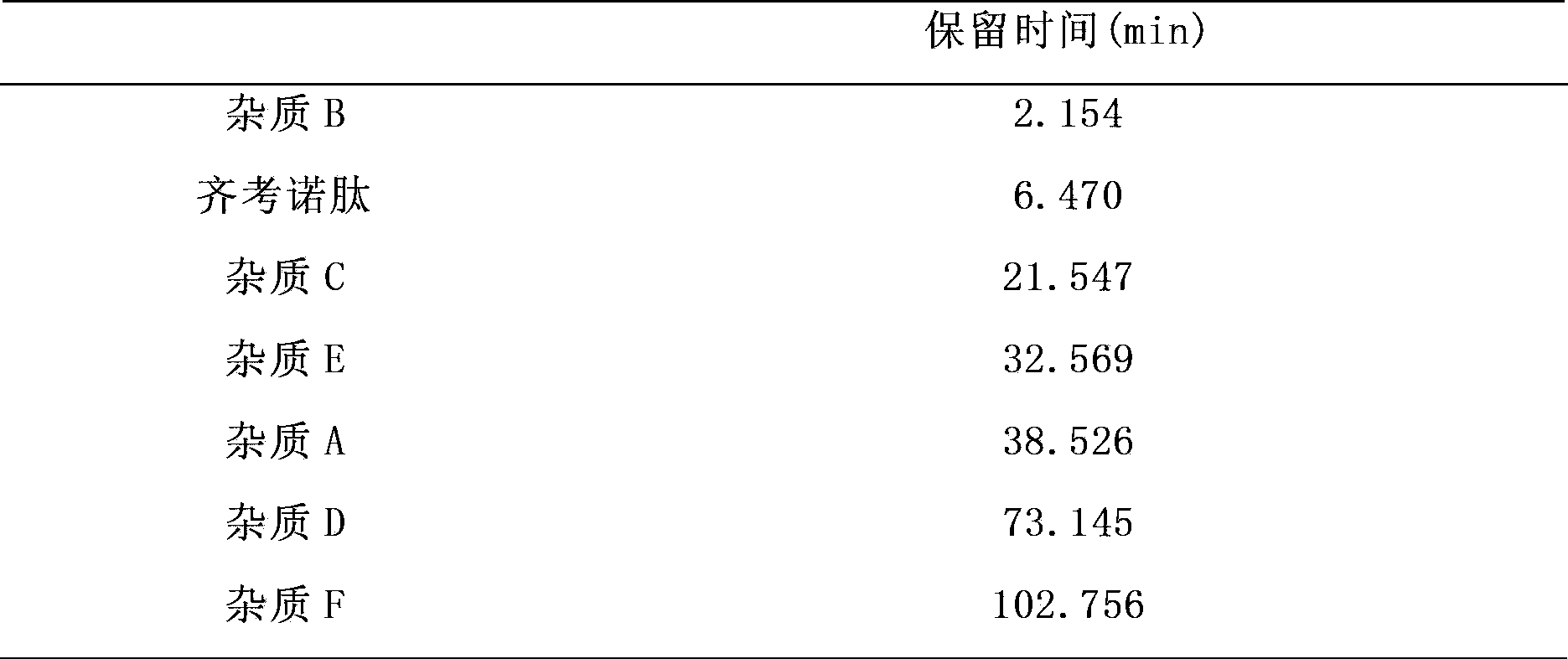
[0062] Get ziconotide and its intermediates and impurities, and use the mobile phase at 0 minutes to dissolve and prepare a test sample solution with a concentration of 1 mg / mL. Set the flow rate to 1.0ml / min, the detection wavelength to 230nm, a...
Embodiment 2
[0067] 1. Instrument conditions
[0068] High performance liquid chromatography: Waters2695
[0069] Chromatographic column: Kromasil C18 (150mm×4.6mm);
[0070] Flow rate: 1.0ml / min
[0071] Column temperature: 0°C
[0072] Injection volume: 20μl
[0073] Mobile phase: mobile phase A is 0.01M potassium dihydrogen phosphate aqueous solution (triethylamine adjusts pH=6.5), mobile phase B is 0.01M potassium dihydrogen phosphate aqueous solution: acetonitrile=40:60 (after the buffer and acetonitrile are fully mixed, Adjust pH=6.5 with triethylamine), see Table 5 below.
[0074]
[0075] Take ziconotide and its impurities, and use appropriate medium to dilute and dissolve to prepare the test sample solution. Set the flow rate to 1.0ml / min, the detection wavelength to 230nm, and the column temperature to 40°C. Take 20 μl of the test sample solution and inject it into the liquid chromatograph. The measurement results are shown in Table 6.
[0076]
[0077]
[0078] Un...
Embodiment 3
[0080] Instrument conditions
[0081] High performance liquid chromatography: Waters2695
[0082] Chromatographic column: Kromasil C18 (150mm×4.6mm);
[0083] Flow rate: 1.0ml / min
[0084] Column temperature: 40°C
[0085] Injection volume: 20μl
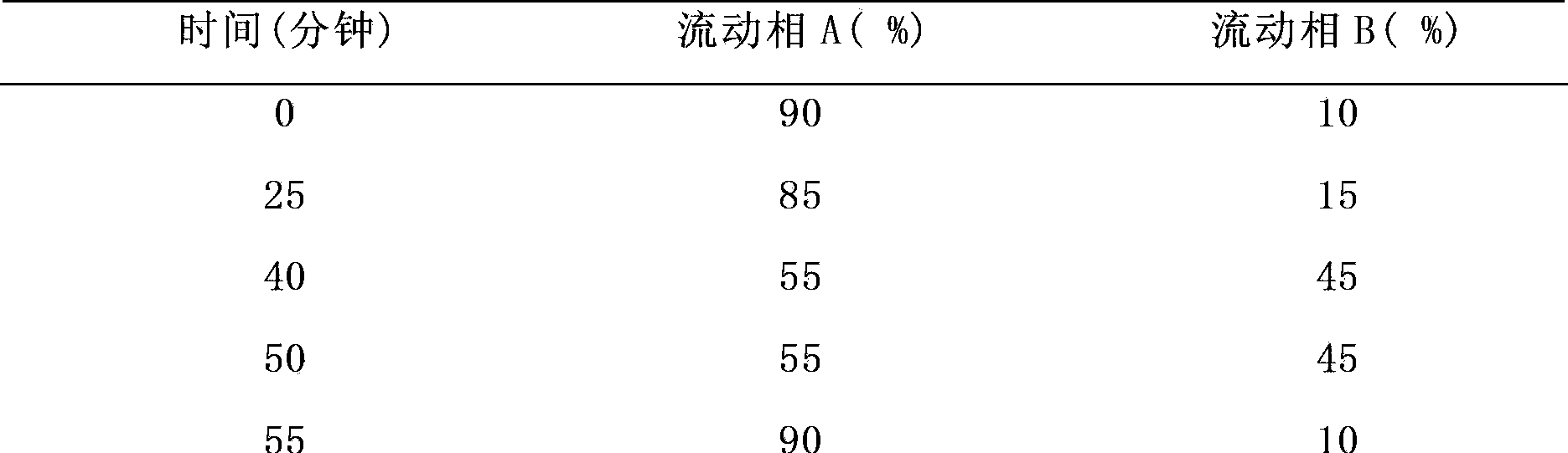
[0086] Mobile phase: mobile phase A is 0.01M sodium dihydrogen phosphate aqueous solution: acetonitrile=85:15 (after the buffer and acetonitrile are fully mixed, adjust the pH to 6.5 with triethylamine), mobile phase B is 0.01M sodium dihydrogen phosphate aqueous solution: Acetonitrile=50:50 (after the buffer solution and acetonitrile are fully mixed, adjust the pH to 6.5 with triethylamine), see Table 7 below.
[0087]
[0088] Take ziconotide and its impurities, and use appropriate medium to dilute and dissolve to prepare the test sample solution. Set the flow rate to 1.0ml / min, the detection wavelength to 230nm, and the column temperature to 40°C. Take 20 μl of the test sample solution and inject it into the liquid chromat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap