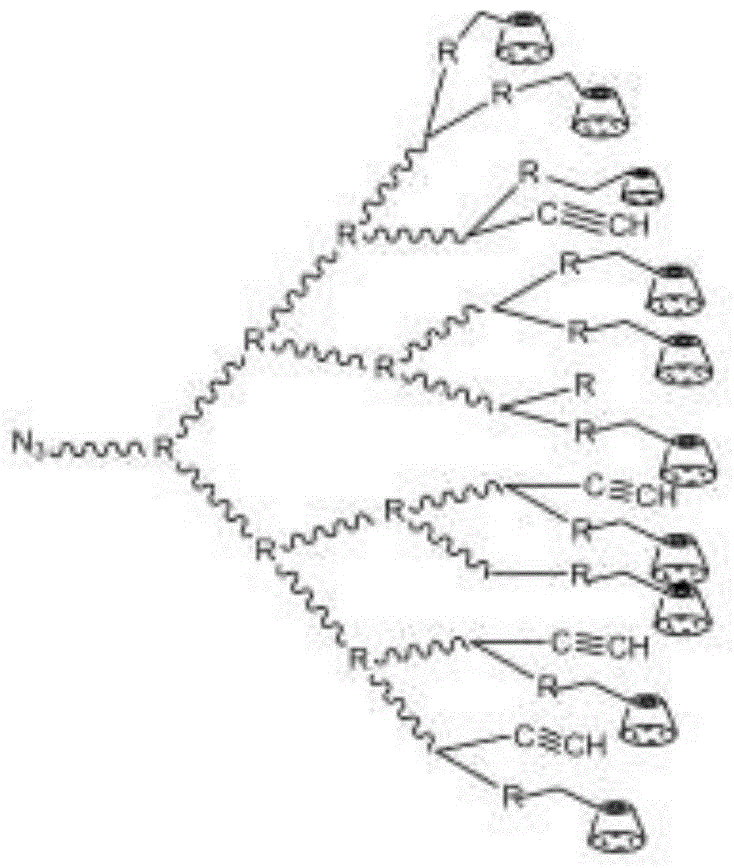
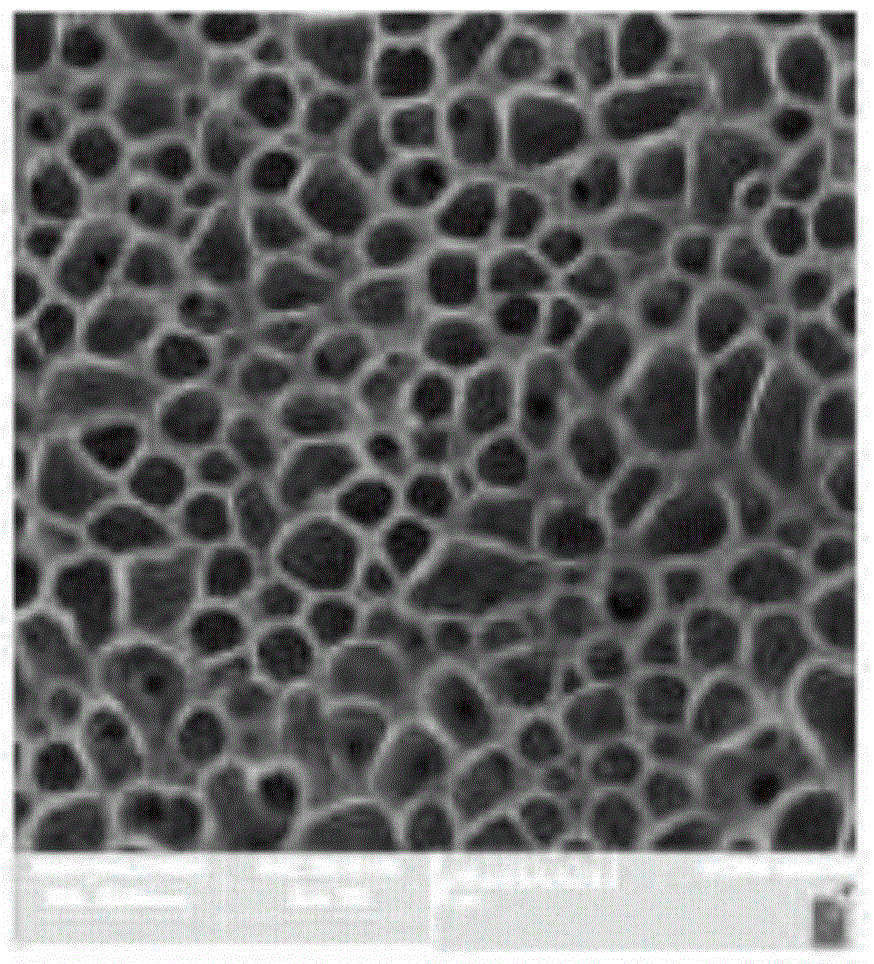
Preparation method of cyclodextrin functionalized long-chain hyperbranched polystyrene porous membrane
A polystyrene and cyclodextrin technology, which is applied in the field of preparation of long-chain hyperbranched polystyrene porous membranes, can solve the problems of low degree of membrane functionalization, complex processing technology, and poor controllability of molecular structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add propynyl alcohol (2.9g, 52mmol), 2-bromosuccinic acid (1028mg, 5.2mmol) and p-toluenesulfonic acid (50mg) successively in dry one-necked flask, and add 10ml toluene as solvent, then in 60 The reaction was carried out under the condition of ℃ for 36h. After the reaction was over, toluene was distilled off under reduced pressure and dissolved in dichloromethane, then washed four times with NaOH solution and three times with distilled water to obtain a yellow liquid, adding anhydrous MgSO 4 Dry and stir for 1h to 2h, and finally remove dichloromethane by vacuum distillation to obtain a yellow viscous liquid, which is 2-bromosuccinic acid dipropynyl alcohol ester.
[0044] Styrene (2080 mg, 10 mmol), dipropynyl 2-bromosuccinate (54.6 mg, 0.2 mmol), bipyridine (93.6 mg, 0.6 mmol) and cuprous bromide ( 28.9mg, 0.2mmol), after completely dissolving, use Schlenk technology to remove the dissolved oxygen in the reaction flask, then react at 110°C under sealed conditions unt...
Embodiment 2
[0051] Add propynyl alcohol (5.8g, 104mmol), 2-bromosuccinic acid (2056mg, 10.4mmol) and p-toluenesulfonic acid (100mg) successively in dry one-necked flask, and add 20ml toluene as solvent, then in 60 The reaction was carried out under the condition of ℃ for 36h. After the reaction was over, toluene was distilled off under reduced pressure and dissolved in dichloromethane, then washed four times with NaOH solution and three times with distilled water to obtain a yellow liquid, adding anhydrous MgSO 4 Dry and stir for 1h to 2h, and finally remove dichloromethane by vacuum distillation to obtain a yellow viscous liquid, which is 2-bromosuccinic acid dipropynyl alcohol ester.
[0052] In a dry Schlenk tube were added styrene (4160 mg, 20 mmol), dipropynyl 2-bromosuccinate (109.2 mg, 0.4 mmol), bipyridine (187.2 mg, 1.2 mmol) and cuprous bromide ( 57.8mg, 0.4mmol), after completely dissolving, use Schlenk technology to remove the dissolved oxygen in the reaction bottle, then rea...
Embodiment 3
[0059] Add propynyl alcohol (8.7g, 156mmol), 2-bromosuccinic acid (3084mg, 15.6mmol) and p-toluenesulfonic acid (150mg) successively in dry one-necked flask, and add 30ml toluene as solvent, then in 60 The reaction was carried out under the condition of ℃ for 36h. After the reaction was over, toluene was distilled off under reduced pressure and dissolved in dichloromethane, then washed four times with NaOH solution and three times with distilled water to obtain a yellow liquid, adding anhydrous MgSO 4 Dry and stir for 1h to 2h, and finally remove dichloromethane by vacuum distillation to obtain a yellow viscous liquid, which is 2-bromosuccinic acid dipropynyl alcohol ester.
[0060] In a dry Schlenk tube were added styrene (6240 mg, 30 mmol), dipropynyl 2-bromosuccinate (163.8 mg, 0.6 mmol), bipyridine (280.8 mg, 1.8 mmol) and cuprous bromide ( 86.7mg, 0.6mmol), after completely dissolving, use Schlenk technology to remove the dissolved oxygen in the reaction flask, then reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com