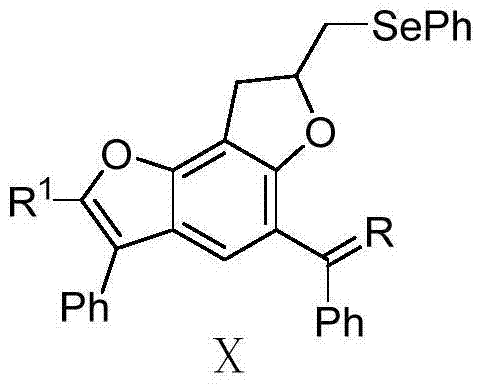
Selenium-containing benzobisfuran type compounds and preparation and applications thereof
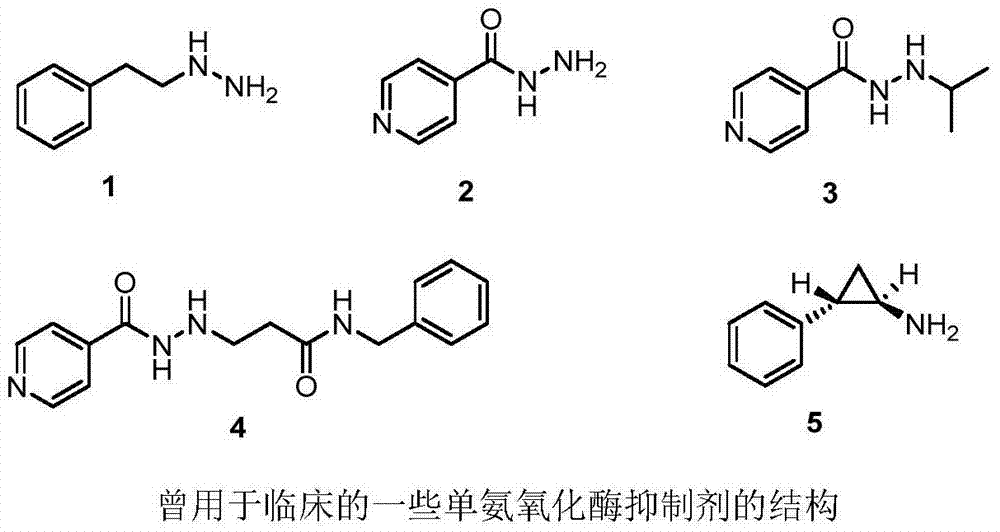
The technology of benzobisfuran and compound is applied in the application field of preparing monoamine oxidase inhibitor, and can solve the problems of monoamine oxidase with single action effect and large toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
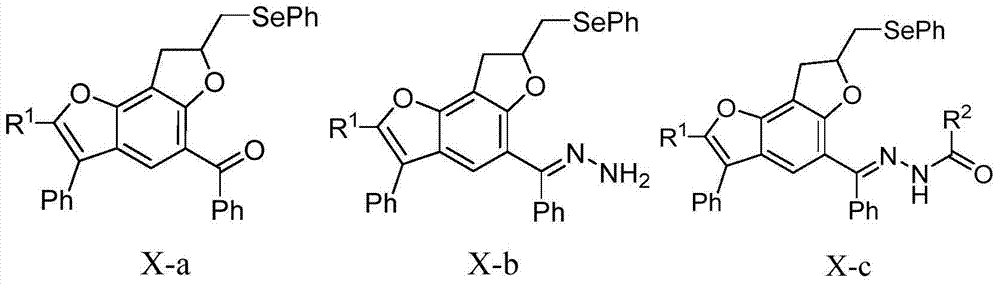
[0049] Example 1 Synthesis of benzobisfuran compound X-a-1
[0050]
[0051] (1) Put 0.513.5g of compound I (1.0mmol) into a round-bottomed flask equipped with a magnetic stirrer and a reflux condenser and dissolve it with 10mL of DMF, then add 0.346g (2.5mmol) of potassium carbonate, and react for ten minutes at room temperature Afterwards, (1.2mmol, 0.164g) bromoacetone was added, and the stirring reaction was continued for 5h. After TLC detected that the reaction was complete, 30 mL of dichloromethane was added, and then washed with a large amount of water (80 mL×5) until DMF was removed, the organic phase was separated, dried with anhydrous magnesium sulfate, concentrated, and subjected to thin-plate chromatography (E / P[ethyl acetate Esters: Petroleum ether] = 1:5.) The benzofuran compound II-1 was isolated with a yield of 80%.
[0052] (2) Dissolve the benzofuran compound II-1 (0.8mmol) obtained in step 1 in 15mL of toluene, and slowly add 0.146g (0.96mmol) of 1,8-dia...
Embodiment 2
[0054] Example 2 Synthesis of benzobisfuran compound X-a-2
[0055]
[0056] (1), drop 0.513.5g compound I (1.0mmol) into the round-bottomed flask equipped with magnetic stirrer, reflux condenser and dissolve it in 10mLDMF, add 0.276g (2.0mmol) potassium carbonate again, at room temperature After reacting for ten minutes, ethyl bromoacetate (1.5mmol, 0.251g) was added, and the stirring reaction was continued for 3.5h. After the reaction was complete as detected by TLC, the follow-up treatment was as in step 1 of Example 1, and a white solid benzofuran compound II-3 was isolated with a yield of 89%.
[0057] (2), the benzofuran compound II-3 (0.89mmol) obtained in step 1 was dissolved in 15mL of toluene, and the subsequent steps were as in example 1, step 2, to obtain the selenium-containing benzofuran compound X-a-2 with a yield of 78 %, the structure is characterized as follows:
[0058] 1 H NMR (400MHz, CDCl 3 )δ7.80-7.78(m,2H),7.63(s,1H),7.57-7.49(m,5H),7.47-7.41(m,5...
Embodiment 3
[0059] Example 3 Synthesis of benzobisfuran compound X-a-3
[0060]
[0061] (1), drop 0.513.5g compound I (1.0mmol) into the round-bottomed flask equipped with magnetic stirrer, reflux condenser and dissolve it in 10mLDMF, add (2.5mmol, 0.346g) potassium carbonate again, room temperature After reacting for ten minutes, 0.106 g (1.4 mmol) of acetonitrile was added, and the stirring reaction was continued for 6 h. After the reaction was detected by TLC, the follow-up treatment was as in step 1 of Example 1, and benzofuran compound II-3 was isolated with a yield of 75%.
[0062] (2), the benzofuran compound II-3 (0.75mmol) obtained in step 1 was dissolved in 15mL of toluene, and the subsequent steps were as in step 2 of Example 1 to obtain the selenium-containing benzofuran compound X-a-3 with a yield of 70 %, the structure is characterized as follows:
[0063] 1 H NMR (400MHz, CDCl 3 )δ7.89-7.68(m,7H),7.65(s,1H),7.64-7.49(m,5H),7.46-7.38(m,3H),5.19-5.13(m,1H),3.72-3.66( ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap