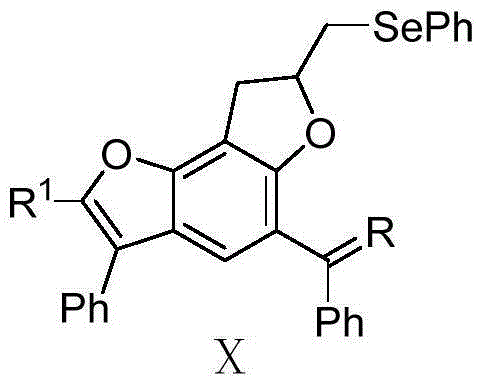
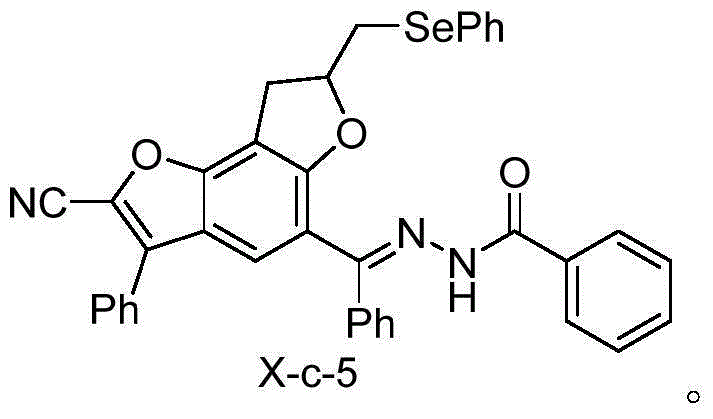
A kind of selenium-containing benzobisfuran compound and its preparation and application
The technology of benzobisfuran and compound is applied in the application field of preparing monoamine oxidase inhibitor, and can solve the problems of single action effect of monoamine oxidase, large toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
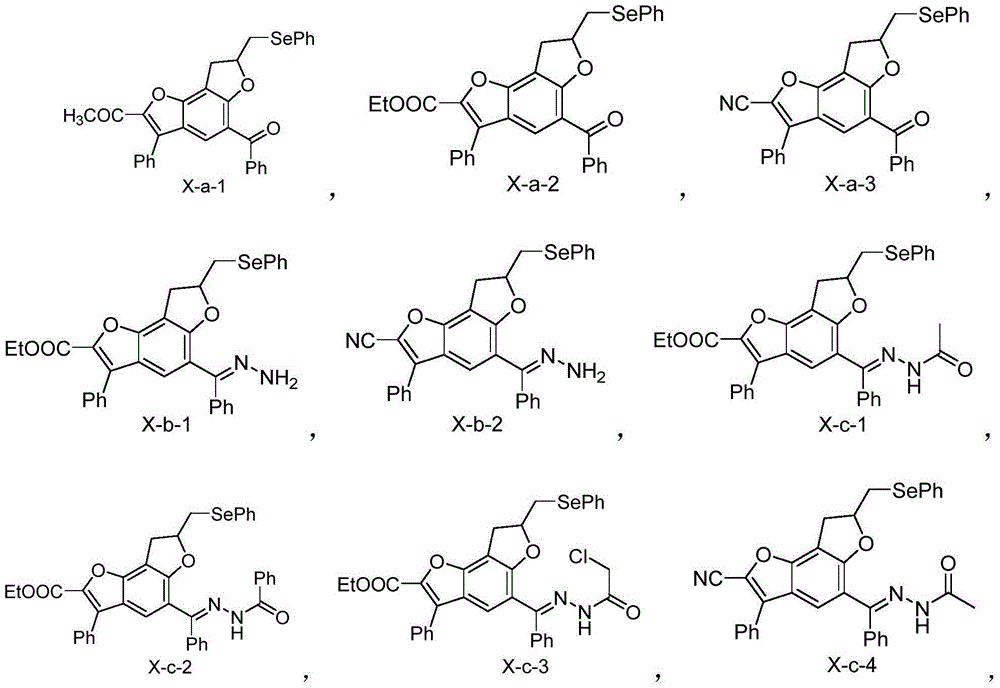
[0049] The synthesis of embodiment 1 benzobisfuran compound X-a-1
[0050]
[0051] (1) Put 0.513.5g of Compound I (1.0mmol) into a round-bottomed flask equipped with a magnetic stirrer and a reflux condenser and dissolve it with 10mL of DMF, then add 0.346g (2.5mmol) of potassium carbonate, and react at room temperature for ten minutes , Add (1.2mmol, 0.164g) bromoacetone, and continue stirring for 5h. After TLC detected that the reaction was complete, 30 mL of dichloromethane was added, and then washed with a large amount of water (80 mL×5) until DMF was removed, the organic phase was separated, dried with anhydrous magnesium sulfate, concentrated, and subjected to thin-plate chromatography (E / P[ethyl acetate Esters: Petroleum ether] = 1:5.) The benzofuran compound II-1 was isolated with a yield of 80%.
[0052] (2) Dissolve the benzofuran compound II-1 (0.8mmol) obtained in step 1 in 15mL of toluene, and slowly add 0.146g (0.96mmol) of 1,8-diazacyclo[5,4,0] dropwise Un...
Embodiment 2
[0054] The synthesis of embodiment 2 benzobisfurans compound X-a-2
[0055]
[0056] (1), drop 0.513.5g compound I (1.0mmol) into the round-bottomed flask equipped with magnetic stirrer, reflux condenser and dissolve it in 10mLDMF, add 0.276g (2.0mmol) potassium carbonate again, at room temperature After reacting for ten minutes, ethyl bromoacetate (1.5mmol, 0.251g) was added, and the stirring reaction was continued for 3.5h. After the reaction was complete as detected by TLC, the follow-up treatment was as in step 1 of Example 1, and a white solid benzofuran compound II-3 was isolated with a yield of 89%.
[0057] (2), the benzofuran compound II-3 (0.89mmol) obtained in step 1 was dissolved in 15mL of toluene, and the subsequent steps were as in example 1, step 2, to obtain the selenium-containing benzofuran compound X-a-2 with a yield of 78 %, the structure is characterized as follows:
[0058] 1 HNMR (400MHz, CDCl 3 )δ7.80-7.78(m,2H),7.63(s,1H),7.57-7.49(m,5H),7.47-7...
Embodiment 3
[0059] The synthesis of embodiment 3 benzobisfuran compounds X-a-3
[0060]
[0061] (1), drop 0.513.5g compound I (1.0mmol) into the round-bottomed flask equipped with magnetic stirrer, reflux condenser and dissolve it in 10mLDMF, add (2.5mmol, 0.346g) potassium carbonate again, room temperature After reacting for ten minutes, 0.106 g (1.4 mmol) of acetonitrile was added, and the stirring reaction was continued for 6 h. After the reaction was detected by TLC, the follow-up treatment was as in step 1 of Example 1, and benzofuran compound II-3 was isolated with a yield of 75%.
[0062] (2), the benzofuran compound II-3 (0.75mmol) obtained in step 1 was dissolved in 15mL of toluene, and the subsequent steps were as in step 2 of Example 1 to obtain the selenium-containing benzofuran compound X-a-3 with a yield of 70 %, the structure is characterized as follows:
[0063] 1 HNMR (400MHz, CDCl 3 )δ7.89-7.68(m,7H),7.65(s,1H),7.64-7.49(m,5H),7.46-7.38(m,3H),5.19-5.13(m,1H),3.72...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com